
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Show full and complete procedure HANDWRITTEN only. Please answer a) b) and c). Note they are subparts of the same question
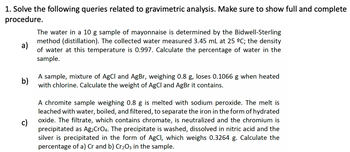
Transcribed Image Text:1. Solve the following queries related to gravimetric analysis. Make sure to show full and complete
procedure.
a)
The water in a 10 g sample of mayonnaise is determined by the Bidwell-Sterling
method (distillation). The collected water measured 3.45 mL at 25 ºC; the density
of water at this temperature is 0.997. Calculate the percentage of water in the
sample.
b)
A sample, mixture of AgCl and AgBr, weighing 0.8 g, loses 0.1066 when heated
with chlorine. Calculate the weight of AgCl and AgBr it contains.
c)
A chromite sample weighing 0.8 g is melted with sodium peroxide. The melt is
leached with water, boiled, and filtered, to separate the iron in the form of hydrated
oxide. The filtrate, which contains chromate, is neutralized and the chromium is
precipitated as Ag₂CrO4. The precipitate is washed, dissolved in nitric acid and the
silver is precipitated in the form of AgCl, which weighs 0.3264 g. Calculate the
percentage of a) Cr and b) Cr₂O3 in the sample.
Expert Solution
arrow_forward
Step 1
We need to find the percentage of water using the given data.
Volume of water collected =3.45 mL and the density of water is 0.997 g/mL
Step by stepSolved in 8 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Edit View History Bookmarks Profiles Tab Window Help ome File d Dashboard ALEKS - Reyna Garcia - Lear Watch Gilmore Girls | Netflix A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IVDWKW_BBZZ16tTytly4Fcfu6zOtC Spotify Web Playe... M Common Ethical D... O CHEMICAL REACTIONS Writing a chemical equation from a description of the reaction Solid potassium and chlorine gas combine to produce solid potassium chloride. Write a balanced chemical equation for this reaction. Explanation Check FEB 2arrow_forwardAutoSave Off CHML 1045 A6 Assignment (1) - Word O Search Danielle Hubbard DH File Home Design Layout References Mailings Review View Help A Share P Comments Insert Draw O Find - - 12 - A A Aav A 三 处T Arial AaBbCcDc AaBbCcDc AaBbCcI AaBbC AaBbCcC Replace Paste BIU - ab x, x A - er A I Normal T No Spac. 1 Table Pa. Heading 1 Heading 2 Dictate Sensitivity Editor Reuse A Select v Files Clipboard Paragraph Styles Sensitivity Reuse FilesA Font Editing Voice Editor L results. Molarity (M) of NaOH (from the bottle of NaOH): 0.204 mol/L Titration Number 3 4 34.44 mL 0.50ML Final Volume buret reading (mL NAOH) 34.00 33.85 mL 0.50mL 33.80 mL 0.50mL 0.50mL Initial Volume buret reading (mL NaOH) Volume NaOH used in titration (mL) = Final Volume buret reading (mL NAOH) - mL Initial Volume buret reading (mL NaOH) 33.94 For calculations multiply mL by 10-3 to convert mL toL Molarity (M or mol/L) NaOH from the bottle of NaOH 0.204 mol/L 10.0 mL 10.0mL 0.204 0.204 0.204 mol/L 10.0 mL 10.0 mL mol/L mol/L…arrow_forward10,11arrow_forward
- (0) Mechanism NaCN скоконarrow_forwardWhy does the tanker crush? Step 1. Observe the giff of the tanker. Step 2. Identify the variables that may be involved and record on CER form in OneNote. Step 3. Make a Claim and add it to form in OneNote. Step 4: Support your claim with evidence (mathematical, diagrammatic, graphically, and texual (observations)). Step 5. Construct an argument on the phenomenon from the giff. (NOTI ou may copy the gill right into your notebook.) Tanker crush from Gifferearrow_forwardHome 101 Chem 101 My Questions bartleby X (274) Banda Carnaval - Sueñ X X X app.101edu.co Unofficial Transcript... Oregon Scholarship.... Welcome to the OS... myClackamas Login Document Require... Apps WLogon Home FAFSA on t... The National Societ... > Submit Question 5 of 20 If 450 g of magnesium hydroxide is dissolved in water to make 6.5 L of solution, what is the concentration in mM? mM 2 1 4 5 6 с 7 8 +- 0 x 100 5:05 PM Type here to search о ENG 11/21/2019 LOarrow_forward
- Help 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forwardI need help with part D pleasearrow_forwardPls answer all 7 questions. I really need it and I don't understandarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY