
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
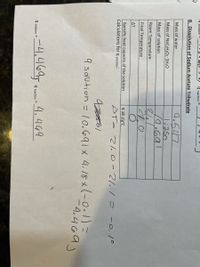

Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use 1 decimal point for all atomic masses. 2.00 g of H2(g) are reacted with 32.0 g of O2(g) by the following reaction 2H2(g) + O2(g) --> 2H2O(g) What is the limiting reagent? H2(g) O2(g) Based on the limiting reagent, fill in the following ICE Table using moles. Enter any zero values as 0.00. H2 O2 H2O Initial Change Final How much of the excess reagent remains (in grams)?arrow_forwardWhen solutions of silver nitrate and sodium chloride are mixed, silver chloride precipitates out of solution according to the equation AGNO3 (aq) + NaCI(aq)→AgCl(s) + NaNO3(aq) Part A What mass of silver chloride can be produced from 1.69 L of a 0.214 solution of silver nitrate? Express your answer with the appropriate units. • View Available Hint(s) ? mass of AgCl = Value Units Submit Part B The reaction described in Part A required 3.48 L of sodium chloride. What is the concentration of this sodium chloride solution? Express your answer with the appropriate units. • View Available Hint(s) HA ? Value Units Submitarrow_forwarda laccd sign in - Search ||| tab hift T 0 caps lock % esc O CHEMICAL REACTIONS Percent yield of chemical reactions Be sure your answer has the correct number of significant digits in it. Explanation K- →1 V Liquid octane (CH₂(CH₂) CH₂) reacts with gaseous oxygen gas (0₂) to produce gaseous carbon dioxide (CO₂) and gaseous water (H₂O). If 10.9 g of water is produced from the reaction of 27.41 g of octane and 176.6 g of oxygen gas, calculate the percent yield of water. m X ? https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmym Type here to search A 2 Z P Check ALEKS W S # X * 3 X alt E *** f4 $ D 70 4 C X R % 100 Mc Graw He f6 T V McGraw-Hill Education Campus X 4- G (0) 17 B Y ♫+ & 7 H no f8 © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Access a ? KAA * J A ALEKS-Shushanik Babayan - Le X + 96sW-8PcvpF38ZsC... A N fg 3 K f10 0/5 L f12 93°F insert alt Shush 11 prt sc ] bac pause ctriarrow_forward
- Part A Hydrobromic acid dissolves solid iron according to the following reaction: What mass of HBr (in g) would you need to dissolve a 3.6-g pure iron bar on a padlock? Fe(s) + 2HB1(aq) → FeBr2 (aq) + H2(g) Express your answer using two significant figures. • View Available Hint(s) Hνα ΑΣφ m = Submit Part B What mass of H2 would be produced by the complete reaction of the iron bar? Express your answer using two significant figures. • View Available Hint(s) Πνα ΑΣφ ? m =arrow_forwardQuestion 16 ons What mass of nitrogen gas (N,) occupies a volume of 14.5L at 33 °C and 1.30 atm? Type your answer in the space provided and show your work on a separate sheet of paper. Edit View Insert Format Tools Tablearrow_forwardNeed thesearrow_forward
- 101 Chem101 G Balance the following chemical b My Questions | bartleby X + app.101edu.co E Apps M Gmail YouTube Мaps VitalSource Books... Question 16 of 24 Submit Balance the following chemical equation (if necessary): Na*(aq) + CI (aq) + Ag*(aq) + NO: (aq) → Na*(aq) + AgCI(s) + NO: (aq) |4– D2+ N3+ 4+ 1 2 3 4 6. 7 8 9 04 (s) (1) (g) (aq) CI Ag NO Na Cr Ar fishes lab.pdf Early Chrodates La...pdf Show all PDF PDF ... 2.arrow_forwardA 0.693 g0.693 g sample of a metal, M, reacts completely with sulfuric acid according to M(s)+H2SO4(aq)⟶MSO4(aq)+H2(g)M(s)+H2SO4(aq)⟶MSO4(aq)+H2(g) A volume of 269 mL269 mL of hydrogen is collected over water; the water level in the collecting vessel is the same as the outside level. Atmospheric pressure is 756.0 Torr, and the temperature is 25 °C. The vapor pressure of water at 25 °C is 23.8 Torr. Calculate the molar mass of the metal.arrow_forward50.020 g sucrose were used to prepare 300.0 mL of solution (sp. gr. = 1.17). What is the % by mass of sucrose in the solution?arrow_forward
- A student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation. kJ 0.060 mol I= ? molarrow_forwardWhen an ionic solid dissolves in water, water molecules attract the ions causing them to dissociate orcome apart. The resulting dissolved ions are electrically charged particles that allow the solution toconduct electricity. The following chemical equations represent this phenomenon:NaCl (s) → Na+ (aq) + Cl-(aq)Na2CO3 (s) → 2Na+(aq) + CO32- (aq)Write a similar balanced chemical equation for the other four strong electrolytes in the data table NaHCO3 KNO3NH4Cl HClarrow_forwarda For the reaction СаBra (aq) + K,SO4 (ag) write the balanced formula equation. (Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank. If no reaction occurs, leave all boxes blank and click on Submit.) СаBr, (aq) K,SO,(aq) CaSO,(s) | 2KC1(aq)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY