
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
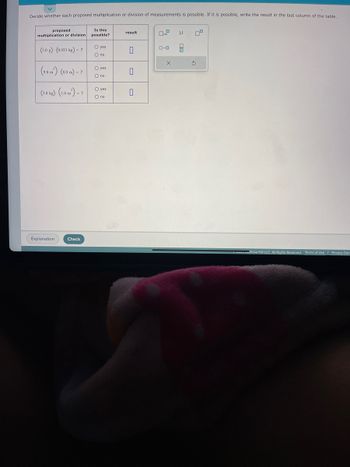
Transcribed Image Text:Decide whether each proposed multiplication or division of measurements is possible. If it is possible, write the result in the last column of the table.
proposed
multiplication or division
(3.0 g). (0.033 kg) - ?
(9.0 m)- (8.0 m) - ?
(3.0 kg)-(1.0 m²) - ?
Explanation.
Check
Is this
possible?
O yes
O no
O yes
O no
O yes
O no
result
0
0
0
O μ
8
0.0
X
Ś
eveu Graw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cent
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemist prepares a solution of mercury(II) iodide (Hgl,) by measuring out 0.0051 µmol of mercury(II) iodide into a 100. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's mercury(II) iodide solution. Be sure your answer has the correct number of significant digits. mol dlo Larrow_forwardA lab prepared a solution of sulfuric acid with a volume of 5.0x10-4 m3. The solution is further concentrated to the half of the original volume, which is used to neutralize a potassium hydroxide solution. The potassium hydroxide solution has a volume of 30 mL, contains 30 g water, and has a density of 1.0141 g/cm3. What is the molarity of sulfuric acid in the concentrated solution? (The final answer keeps 4 digits after decimal. Please do NOT use scientific notation.)arrow_forwardHow many liters of a 0.209 M KI solution is needed to completely react with 2.43 g of Cu(NO:)2 according to the balanced chemical reaction: 2 Cu(NO:):(aq) + 4 KI(aq) - 2 Cul(aq) + I:(s) + 4 KNO:(aq) STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 4 6.022 x 1023 0.0310 166 1 0.209 0.124 2.43 187.57 0.0620 0.00542 g Cu(NO.)2 g KI mL KI M KI mL Cu(NO:)2 mol KI L KI mol Cu(NO:) L Cu(NO:)arrow_forward
- Hi! Could you please help me into putting the formula into the format as above? Thank you!arrow_forward5. Sodium bicarbonate (NaHCO3) is used to neutralize acids to form sodium chloride, a salt, carbon dioxide gas, and water. Normally sodium bicarbonate is added until the fizzing ceases, at which point no more CO2 (g) is produced. NaHCO3 (s) + HCl (aq) NaCl (aq) + CO2 (g) + H2O (1) If 150. mL of 3.0 M HCl is spilled on a lab bench, what is the minimum mass of sodium bicarbonate that must be used to neutralize the acid?arrow_forward1-1. The raw water supply for a community contains 18 mg/L total particulate matter. It is to be treated by addition of 60 mg alum (Al2(SO4)3-14H;O) per liter of water treated. Essentially, all the added alum precipi- tates represented by the following reaction: Al2(SO4)3 · 14H20 → 2 Al(OH);(s) + 3So,- + 6H* + 8H2O For a total flow of 7500 m/d, compute the daily alum requirement, the total concentration of suspended sol- ids in the water following alum addition, and the daily load of particulate solids requiring disposal (including both those initially present and those formed during treatment).arrow_forward
- Part A Hydrobromic acid dissolves solid iron according to the following reaction: What mass of HBr (in g) would you need to dissolve a 3.6-g pure iron bar on a padlock? Fe(s) + 2HB1(aq) → FeBr2 (aq) + H2(g) Express your answer using two significant figures. • View Available Hint(s) Hνα ΑΣφ m = Submit Part B What mass of H2 would be produced by the complete reaction of the iron bar? Express your answer using two significant figures. • View Available Hint(s) Πνα ΑΣφ ? m =arrow_forward5) If all the NaHCO3 in bag 2 reacted, calculate the number of moles of gas produced? tates) E Focusarrow_forwardGive the mass of the solute and mass of the solvent for 1.30 LL of a solution that is 17.0 %% of Pb(NO3)2Pb(NO3)2 by mass (the density of the solution is 1.16 g/mLg/mL), starting with solid solute. Express your answers using three significant figures. Enter your answers numerically separated by a comma.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY