
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Please note all assumptions and when appendix values are used.
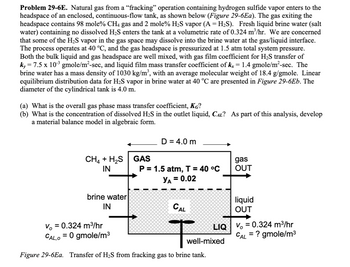
Transcribed Image Text:Problem 29-6E. Natural gas from a "fracking" operation containing hydrogen sulfide vapor enters to the
headspace of an enclosed, continuous-flow tank, as shown below (Figure 29-6Ea). The gas exiting the
headspace contains 98 mole% CH4 gas and 2 mole% H₂S vapor (A = H₂S). Fresh liquid brine water (salt
water) containing no dissolved H₂S enters the tank at a volumetric rate of 0.324 m³/hr. We are concerned
that some of the H₂S vapor in the gas space may dissolve into the brine water at the gas/liquid interface.
The process operates at 40 °C, and the gas headspace is pressurized at 1.5 atm total system pressure.
Both the bulk liquid and gas headspace are well mixed, with gas film coefficient for H₂S transfer of
ky = 7.5 x 10-³ gmole/m²-sec, and liquid film mass transfer coefficient of kx = 1.4 gmole/m²-sec. The
brine water has a mass density of 1030 kg/m³, with an average molecular weight of 18.4 g/gmole. Linear
equilibrium distribution data for H₂S vapor in brine water at 40 °C are presented in Figure 29-6Eb. The
diameter of the cylindrical tank is 4.0 m.
(a) What is the overall gas phase mass transfer coefficient, KG?
(b) What is the concentration of dissolved H₂S in the outlet liquid, CÂ? As part of this analysis, develop
a material balance model in algebraic form.
CH4 + H₂S GAS
IN
brine water
IN
V = 0.324 m³/hr
CAL,O
= 0 gmole/m³
D = 4.0 m
P = 1.5 atm, T = 40 °C
YA = 0.02
CAL
LIQ
well-mixed
Figure 29-6Ea. Transfer of H₂S from fracking gas to brine tank.
gas
OUT
liquid
OUT
V = 0.324 m³/hr
= ? gmole/m³
CAL
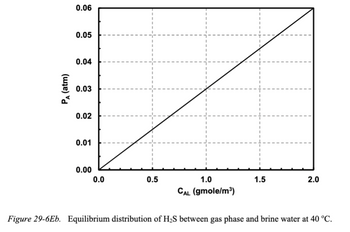
Transcribed Image Text:PA (atm)
0.06
0.05
0.04
0.03
0.02
0.01
0.00
0.0
0.5
1.0
CAL (gmole/m³)
1.5
2.0
Figure 29-6Eb. Equilibrium distribution of H₂S between gas phase and brine water at 40 °C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The