
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
answers given
steps how to reach these please
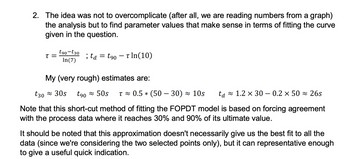
Transcribed Image Text:2. The idea was not to overcomplicate (after all, we are reading numbers from a graph)
the analysis but to find parameter values that make sense in terms of fitting the curve
given in the question.
T =
t90-t30
In(7)
; ta = t90 - Tln (10)
My (very rough) estimates are:
t30≈ 30s t90≈ 50s T≈ 0.5 * (5030) 10s ta 1.2 × 30 – 0.2 × 50≈ 26s
Note that this short-cut method of fitting the FOPDT model is based on forcing agreement
with the process data where it reaches 30% and 90% of its ultimate value.
It should be noted that this approximation doesn't necessarily give us the best fit to all the
data (since we're considering the two selected points only), but it can representative enough
to give a useful quick indication.
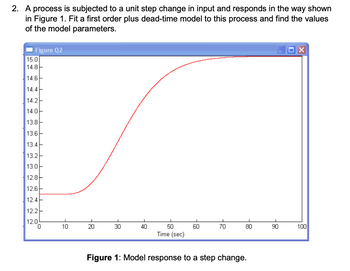
Transcribed Image Text:2. A process is subjected to a unit step change in input and responds in the way shown
in Figure 1. Fit a first order plus dead-time model to this process and find the values
of the model parameters.
Figure Q2
15.0
14.8
14.6
14.4
14.2
14.0
13.8
13.6
13.4-
13.2-
13.0-
12.8
12.6
12.4
12.2
12.0
0
I
10
1
20
30
40
50
Time (sec)
1
60
70
80
Figure 1: Model response to a step change.
90
100
Expert Solution
arrow_forward
Step 1: Given data
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Similar questions
- Answer only when know..exactly otherwise skip it please 1015. What are the key factors in developing a process intensification roadmap?arrow_forwardThank you for you reply I appreciate it. Would you be able to expand on what you did for the integration at the for Part A please?arrow_forwardsummarize Linear regression and population models (chemical/bio engineering)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The