
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
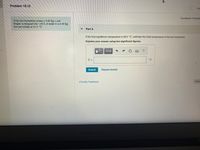
Transcribed Image Text:Problem 19.12
1 of
Constants I Periodic
Ahot iron horseshoe (mass = 0.35 kg ), just
forged, is dropped into 1.45 L of water in a 0.40 kg
iron pot initially at 21.0 °C.
%3D
Part A
If the final equilibrium temperature is 29.0 'C, estimate the initial temperature of the hot horseshoe.
Express your answer using two significant figures.
T =
°C
Submit
Request Answer
Provide Feedback
Next
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 18.9 Please help me answer this physics questionarrow_forwardConstants Periodic Table Assume all temperatures to be exact, and neglect significant figures for small changes in dimension. On a warm day (92 °F), an air-filled balloon occupies a volume of 0.300 m³ and has a pressure of 24.0 lb/in?. Part A If the balloon is cooled to 32 °F in a refrigerator while its pressure is reduced to 14.7 lb/in?, what is the volume of the air in the container? (Assume that the air behaves as an ideal gas.) nνα ΑΣφ V = 0.170 m3 Submit Previous Answers Request Answer X Incorrect; Try Again; 6 attempts remainingarrow_forward11arrow_forward
- What is the maximum mass of ethyl alcohol you could boil with 2000 J of heat, starting from 17 °C? Express your answer with the appropriate units. ► View Available Hint(s) μᾶ Value Units ?arrow_forwardPart A Helium gas with a volume of 2.60 L, under a pressure of 0.190 atm and at a temperature of 43.0° C, is warmed until both pressure and volume are doubled. What is the final temperature? Express your answer in degrees Celsius. ? T = 172 °C Submit Previous Answers Request Answer Previous Answers X Incorrect; Try Again; 5 attempts remaining Part B How many grams of helium are there? The molar mass of helium is 4.00 g/mol. Express your answer in grams. V ΑΣφ m =arrow_forwardQuestion #8 pleasearrow_forward
- Perfectly rigid containers each hold n moles of ideal gas, one being hydrogen (H₂) and other being neon (Ne). Part A If it takes 300 J of heat to increase the temperature of the hydrogen by 2.70°C, by how many degrees will the same amount of heat raise the temperature of the neon? Express your answer in degrees Celsius. ATNe= Submit 17 ΑΣΦ Request Answer ? °Carrow_forwardA(n) 65-g ice cube at 0°C is placed in 680 g of water at 28°C. What is the final temperature of the mixture? 27.31 X Your response differs from the correct answer by more than 10%. Double check your calculations. °Carrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON