
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
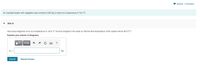
Transcribed Image Text:I Review I Constants
An insulated beaker with negligible mass contains 0.235 kg of water at a temperature of 75.0°C.
Part A
How many kilograms of ice at a temperature of -20.0° C must be dropped in the water so that the final temperature of the system will be 30.0° C?
Express your answer in kilograms.
?
kg
m =
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 11arrow_forwardPerfectly rigid containers each hold n moles of ideal gas, one being hydrogen (H₂) and other being neon (Ne). Part A If it takes 300 J of heat to increase the temperature of the hydrogen by 3.00° C, by how many degrees will the same amount of heat raise the temperature of the neon? Express your answer in degrees Celsius. ATNe = Submit VG ΑΣΦ Request Answer ? °C Qarrow_forwardPart A Helium gas with a volume of 2.60 L, under a pressure of 0.190 atm and at a temperature of 43.0° C, is warmed until both pressure and volume are doubled. What is the final temperature? Express your answer in degrees Celsius. ? T = 172 °C Submit Previous Answers Request Answer Previous Answers X Incorrect; Try Again; 5 attempts remaining Part B How many grams of helium are there? The molar mass of helium is 4.00 g/mol. Express your answer in grams. V ΑΣφ m =arrow_forward
- An insulated beaker with negligible mass contains liquid water with a mass of 0.245 kg and a temperature of 66.4 °C. Part A How much ice at a temperature of -17.8 °C must be dropped into the water so that the final temperature of the system will be 34.0°C? Take the specific heat of liquid water to be 4190 J/kg K, the specific heat of ice to be 2100 J/kg-K, and the heat of fusion for water to be 3.34x105 J/kg. View Available Hint(s) mice = [ΨΕΙ ΑΣΦ 5 kgarrow_forwardWhen a 270-g piece of iron at 160 °C is placed in a 95-g aluminum calorimeter cup containing 250 g of liquid at 10° C, the final temperature is observed to be 35 °C. The value of specific heat for iron is 450 J/kg·C°, and for aluminum is 900 J/kg · C°. Part A Determine the specific heat of the liquid. Express your answer using two significant figures. VO AE ? J C = kg-C° Submit Request Answerarrow_forwardOne end of a 70-cm-long copper rod with a diameter of 2.0 cm is kept at 420 °C, and the other is immersed in water at 24 °C. Part A Calculate the heat conduction rate along the rod. Express your answer to two significant figures and include the appropriate units. t μA = Value Submit Units and Previous Answers Request Answer ?arrow_forward
- An ideal gas at 20°C consists of 2.2 x 1022 atoms. 3.2 J of thermal energy are removed from the gas. Part A What is the new temperature in °C? Express your answer in degrees Celsius. DA ΑΣφ T = °C Submit Request Answerarrow_forwardPerfectly rigid containers each hold n moles of ideal gas, one being hydrogen (H₂) and other being neon (Ne). Part A If it takes 300 J of heat to increase the temperature of the hydrogen by 2.70°C, by how many degrees will the same amount of heat raise the temperature of the neon? Express your answer in degrees Celsius. ATNe= Submit 17 ΑΣΦ Request Answer ? °Carrow_forwardA physicist uses a cylindrical metal can 0.350 m high and 0.090 m in diameter to store liquid helium at 4.22 K; at that temperature the heat of vaporization of helium is 2.09 x 104 J/kg. Completely surrounding the metal can are walls maintained at the temperature of liquid nitrogen, 77.3 K, with vacuum between the can and the surrounding walls. Part A How much helium is lost per hour? The emissivity of the metal can is 0.200. The only heat transfer between the metal can and the surrounding walls is by radiation. Express your answer in gram per hour. Am At Submit ΠΕ ΑΣΦΑΦ Request Answer PZ ? Review | Constants g/harrow_forward
- A 850 g aluminum pan is removed from the stove and plunged into a sink filled with 11.0 L of water at 20.0 °C. The water temperature quickly rises to 24.0 °C. Part A What was the initial temperature of the pan in °C? Express your answer with the appropriate units. Tinitial Submit Part B 11 Tinitial VO V—| ΑΣΦ Request Answer What was the initial temperature of the pan in °F? Express your answer with the appropriate units. VE ΑΣΦ C Ć SALA CO — ? ? °F °Carrow_forwardThe basal metabolic rate is the rate at which energy is produced in the body when a person is at rest. A 68 kg (150 lb) person of height 1.83 m (6.0 ft) would have a body surface area of approximately 1.9 m². Part A What is the net amount of heat this person could radiate per second into a room at 19.0° C (about 66.2°F) if his skin's surface temperature is 31.0° C? (At such temperatures, nearly all the heat is infrared radiation, for which the body's emissivity is 1.0, regardless of the amount of pigment.) Express your answer in watts. ΑΣφ ? Hnet W %D Submit Request Answer Part B Complete previous part(s)arrow_forwardConstanS T Pent Part A An experiment measures the temperature of a 400 g substance while steadily supplying heat to it. The figure shows the results of the experiment. (Figure 1) What is the specific heat of the solid phase? Express your answer to two significant figures and include the appropriate units. ? Value Units C = Submit Request Answer Part B What is the specific heat of the liquid phase? Express your answer to two significant figures and include the appropriate units. ? Value Units C = Submit Request Answer Part C Figure What is the melting temperature? Express your answer to two significant figures and include the appropriate units. T(°C) 80 ? 60 40 T, - Value Units 20 Q (kJ) 40 80 120 160 200 -20 Submit Request Answer -40arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON