
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
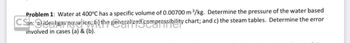
Transcribed Image Text:Problem 1: Water at 400°C has a specific volume of 0.00700 m³/kg. Determine the pressure of the water based
CSn: @ideal gas povaltion: bit the reneralized compressibility chart; and c) the steam tables. Determine the error
involved in cases (a) & (b).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- the pressure drop across the tube bank, and (c) the rate of condensation of steam inside the tubes. Evaluate the air properties at an assumed mean temperature of 35°C and 1 atm. Is this a good assumption?solve this part tooarrow_forwardOn a day when wind is blowing over a lake (T(water) =20°C) at 5 m/s, which side of the air-water interface will be rate-limiting on the flux of Ethyl benzene (i.e. va or vw)?arrow_forward(5)An insulated piston-cylinder device contains 15 L of saturated liquid water at a constant pressure of 950 kPa. Water is stirred by a paddle wheel while a current of 15 A flows for 28 min through a resistor placed in the water. If 64% of the liquid is evaporated during this constant pressure process and the paddle-wheel work amounts to 600 kJ, determine the voltage of the source. Also, show the process on a P-v diagram with respect to saturation lines. H,0 P= constant W. W.arrow_forward
- 4. A mass of 1.7 kg of saturated liquid water is completely vaporized at a constant pressure of 101.325 kPa. Determine (a) the volume change and (b) the amount of energy transferred to the water.arrow_forwardA wet cooling tower is to cool 25 kg/s of cooling water from 40 to 30°C, at a locationin which the atmospheric pressure is 96 kPa. Atmospheric air enters the tower at 20 °C and 70 percent relative humidity, and leaves saturated at 35 °C. Neglecting the power input to the fan, determine (a) the volumetric flow rate of air at the cooling tower inlet and (b) the mass flow rate of make-up water required.arrow_forwardIf solar radiation intensity is 700 W/m² in NJ, how much solar energy arrives at a solar collector with an area of 2 m² in 12 hours? If the solar collector can convert 33% of solar energy collected to electricity, how much electricity (kWh) will be generated?arrow_forward
- answer asap plz and show all workarrow_forwardAir at 150 kPa and 25°C is transported through a 2-inch Schedule 40 commercial steel pipe. Consider that the air behaves as an ideal gas, that the flow is adiabatic and that the Mach number under these conditions of pressure and temperature is 0.35. Calculate the length of the pipe when the air pressure and temperature decrease to 54.375 kPa and -10.6°C.arrow_forwardThe work for irreversible compression of an ideal gas in a piston-cylinder system is the work for reversible compression to the same final pressure. a) higher than b) lower than c) the same as d) We need more informationarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The