
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Transcribed Image Text:◇ Principles of Organic Chemistry
=
Identifying the enantiomer of a simple organic molecule
1GгO16p5...
0/5
Julianna
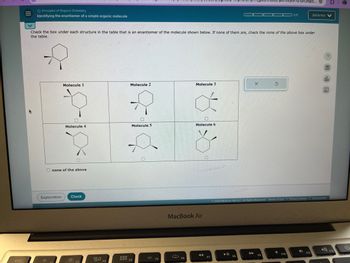
Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under
the table.
olo
Molecule 1
Molecule 2
Molecule 3
X 5
Ar
إلى إلى
1918
Molecule 4
Molecule 5
Molecule 6
none of the above
Explanation
Check
MacBook Air
2024 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility
F12
►11
F10
DOD
esc
80
F2
F3
000 F4
F7
F5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Organic Chemistry HW: CANNOT BE HAND DRAWN 2,6-dimethyloct-2-ene Provide a detailed typed explanation of Stereoisomers show the expanded structure of your molecule. Calculate the maximum number of possible stereoisomers of your molecule using the following formula: Maximum number of possible stereoisomers = 2n (where n= the number of chiral carbons in your molecule). This calculation does not include E- or Z- isomers for any compounds containing double bonds Type or using a computer program "draw" the possible stereoisomers of the molecule. Note that E-, Z- isomers of each stereoisomer are also possible and would not be accounted for by the formula above; draw any E- or Z- isomers.arrow_forwardDetermine (A.) functional groups presents in the molecules and (B) determine the number of chiral carbons in the molecule (Exclude alkane) NH2 HO (Hint: Only six functional groups available)arrow_forwardDetermine (A.) functional groups presents in the molecules and (B) determine the number of chiral carbons in the molecule (Exclude alkane) (Hint: Only 5 functional groups available)arrow_forward
- To preview the image click here State whether the following pairs of structures are the same structure just looked at from a different direction/drawn in a different way OR whether they are structural isomers OR stereoisomers OR completely different structures that are not isomers. Answer for A✓ [Select] Answer for E Answer for C A B C Same Structure Structural Isomers Stereoisomers Completely Different Structures that are not isomers trans-CHBRCHCH₂CH3 나 :: : Brarrow_forwardCannot be hand-drawn - type or computer illustrate Help correct my work - Calculate the maximum number of possible stereoisomers of your molecule using the following formula: ▪ Maximum number of possible stereoisomers = 2n (where n= the number of chiral carbons in your molecule). This calculation does not include E- or Z- isomers for any compounds containing double bonds o illustrate the possible stereoisomers of the molecule.arrow_forwardChiral Carbons print/draw the expanded structure of 4-bromo-4-ethylhept-1-ene. Determine if your molecule contains any chiral carbons. If there are chiral carbons in your molecule, circle or highlight all of them. If your molecule does not contain any chiral carbons explain why none of the carbons are chiral.arrow_forward
- Following is the formula of a simple sugar. Which of the following functional groups is NOT in such a compound? O || OH H OH OH H | H-C-C-C-C-C-C-OH | | | H 1 1 OH H HH carbobyl (C=O) group O hydroxyl (-OH) group O aldehyde (-CHO) group O thiol (-SH) group hparrow_forwardFor example, alanine is a chiral amino acid that has two enantiomers: (+)-alanine and (-)-alanine. These two are optical isomers. NH2 NH2 4. One of the most important properties of chiral molecules in solution is their effect on plane- polarized light, this effect is called optical activity. -C H 2 COOH HOOC CH When an enantiomer rotates a plane-polarized light in the positive direction or clockwise, it is dextrorotary (+ or d), while for a negative direction or counterclockwise, it is levorotary (- or l) (+)-Alanine (-)-Alanine Alanine is a chiral amino acid that has two enantiomers: (+)-alanine and ()-alanine. These two are optical isomers.arrow_forward1b. Draw ALL isomers (including all stereisomers) of trichlorocyclopentane. Indicate which ones are chiral.arrow_forward
- O HYDROCARBONS Naming branched alkanes Name the following organic compounds: CH3 | Explanation compound CH3 I CH₂-CH₂-CH-CH₂ CH₂ - CH₂ - CH3 | CH3-CH₂-C- CH₂ - CH₂ - CH3 | CH3 - CH₂ - CH₂ Check CH3 — CH₂-CH-CH₂ - CH₂ name I 0 X Ś Ⓒ2022 McGraw Hillarrow_forwardFor the following compounds identify and draw freehand structural isomers and/ or stereoisomers where possible. For each isomer, clearly explain what type of isomerism is being shown and how it arises. i) Br2C=CH2 ii) CH3CH2CH2CHOarrow_forwardWhy are these two structures enantiomers? They look more like structural isomers to me.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning