
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Option E is none of the answer also an option, it got cut off
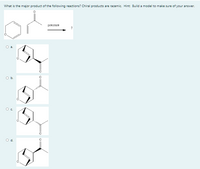
Transcribed Image Text:What is the major product of the following reactions? Chiral products are racemic. Hint Build a model to make sure of your answer.
pressure
Ob.
Od.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free energy of the following chemical reaction: 4Fe (s)+30, (g)→ 2Fe,0, (s) Round your answer to zero decimal places. kJ x10 Continue O2021 McGraw Hill LLC. All Rights Reserved. Termsarrow_forwardPart A transcript When glucose (C6H12O6 (s)) is consumed, it reacts with O₂ gas in the body to produce gaseous carbon dioxide and liquid water. Enter the balanced chemical equation for the reaction. Express your answer as a chemical equation including phases. 0 ΑΣΦ ? A chemical reaction does not occur for this question. Submit Previous Answers Request Answerarrow_forwardI know you don't answer graded questions and I am not looking for you to straight up give me the answer to this but how do I even start? like I said before I know that the highest peak is 16amu which is Oxygen but do I just the vertical bar as a ratio? Like the next bar to the left (15amu?) is like 90% intensity? so like 9:10 ratio? There isn't even a 15amu element so please just give me some idea where to start. The place in the book it tells you to go to read has nothing about this stuff, its about finding empirical formulas from mass or percentages.... please help me!arrow_forward
- Chemistry In an analysis of the content of carbohydrate present in a glycoprotein, the following results were found: 12.6, 11.9, 13.0, 12.7 and 12.5 g of carbohydrate per 100 g of protein. Taking into account that σ is unknown, the confidence interval for the average value at a 90% confidence level of the carbohydrate content is: Select one:to. 12.5 ± 0.4b. 12.5 ± 0.2c. 12.5 ± 0.5d. 12.5 ± 0.3arrow_forwardCan you show how to out this calculation in the calculator? Everytime I do it, I get a different answer.arrow_forward3. Consider a solution containing dipolar molecules each having a positively charged head and a negatively charged tail. In the absence of an electric field the molecules point north (n), south (s), east (e), and west (w) with equal probabilities (distribution shown in Fig. 3a). A different probability distribution (shown in Fig. 3b), however, is found when a field is applied to the solution. P P 14 n e น S 7 16 14 16 n e W s Fig. 3a (field absent) Fig. 3b (field present) a) Determine the direction in which the field has its most positive pole (the polarity of the field). b) In the absence of the field, what is the entropy of the system? c) In the presence of the field, what is the entropy of the system? d) Does the application of the field cause the system to become more ordered or disordered?arrow_forward
- N1₂03 #N₁ und calculate AHF 19.33g Sien #5 02 21.35 N16) + 110 M₂03(S) initial water temps 21.45°2 Finglater temp 24.63 MASS OF GloRutes 6000g SPOF GLORIR 0.44) Sc MASS OF LATER 12000g Spuster 4.18-3/8<arrow_forwardChrome File Edit View History Bookmarks People Tab Window Help Hcc Dashbc x E Buy Es: x G find so x © Periodi x A ALEKS X HUc Chapte x E New m x G conver X 不→ C A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNsIkr7j8P3jH-IQiHqRdYV_6Ux63SypJXz0Coxvwqgg4JkWI7FgD9QGpr.. O GASES O OC D Jacqueline v Using Avogadro's Law Hydrogen gas and nitrogen gas react to form ammonia gas. What volume of ammonia would be produced by this reaction if 7.5 m of nitrogen were consumed? Also, be sure your answer has a unit symbol, and is rounded to 2 significant digits. 圖 中 ロ alo oloarrow_forwardO Course Home b Chemistry Question | bartleby G indivuials with austium good to -> A openvellum.ecollege.com/course.html?courseld=16561285&OpenVellumHMAC=446c877376a9bc7ac878d8ad119f4717#10001 Q * E Apps O Maps E Connect - To Do As... O OCCC Moodle P chem work b help Gmail YouTube Balance Chemical E. II Review | Constants | Periodic Table A carbon atom is chiral if it is bonded to four different groups. For example, CHCIBII is chiral, but CCl,BrI is achiral because some of the bonded groups are the same. If a chiral carbon atom is present, then that molecule has a non-superimposable mirror image called an enantiomer. (Figure 1) Part A Identify all the chiral atoms in the below structure by right-clicking* a chiral atom to bring up a menu that includes "Atom Properties." Click on Atom Properties then click the checked box next to the Map field to clear the checkmark. Then enter "1" in the Map *Mac users: Use an equivalent for right- field box to label that chiral carbon atom. clicking.…arrow_forward
- 7. Don believes that fewer than one-third of the students at his college hold part-time jobs while in school. To investigate this, he randomly surveyed 116 students and found that 32 students held part-time jobs. a. ( - Provide a 90% confidence interval for the population proportion of students that hold a part-time job. b. 1 (a). ;Give the correct interpretation of the interval in part c. Extra Credit: Does this interval support Don's claim? Why?arrow_forwardOWLV2 | Online teaching and X 0mySigTau xb Login | bartleby X M COMM.1113: FUND OF ORA X G what food has c - Google Se X + om/ilrm/takeAssignment/takeCovalentActivity.do?locator=assignment-take D [References] hotogray lenses incorporate small amounts of silver chloride in the glass of the lens. When light hits the AgCl particles, the following reaction occurs: hv AgCl Ag + Cl The silver metal that is formed causes the lenses to darken. The enthalpy change for this reaction is 2.90 × 10² kJ/mol. Assuming all this energy must be supplied by light, what is the maximum vavelength of light that can cause this reaction? Wavelength %3D nm Submit Answer Try Another Version 3 item attempts remaining Previous Nextarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY