
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN: 9781305960060
Author: Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
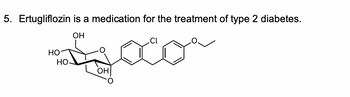
Transcribed Image Text:5. Ertugliflozin is a medication for the treatment of type 2 diabetes.
ОН
НО
НО.
ОН
Cl

Transcribed Image Text:5.2 Based on your identification above, predict the stability of Ertugliflozin under
aqueous acidic and basic conditions. Complete the sentence:
acidic/basic
Ertugliflozin is stable under aqueous
unstable under aqueous
conditions.
5.3
acidic/basic
conditions and
Support your predication by providing arrow-pushing mechanism for the hydrolysis
reaction under the conditions you chose above. Use HA or B as needed.
5.4 Draw the structure of the hydrolysis reaction product(s).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Can you give a solution for Question 5.4 please.
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Can you give a solution for Question 5.4 please.
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 9. Which of the following molecules is the least water soluble? НО. ОН Н НО `NH2 dopamine Н a. НО estradiol c. НО -NH НО ОН b. adrenalin Н HO d. cholesterolarrow_forwardAnsaid and Motrin belong to the group of drugs known as nonsteroidal anti-inflammatory drugs (NSAIDs). Both are only slightly soluble in water, but one is a little more soluble than the other. Which of the drugs has the greater solubility in water?arrow_forwardWhy are antioxidants important to the body? O They increase the likelihood of cancer. O They slow the oxidation process. They help to break down glucose. O They help blood flow.arrow_forward
- When Fructose (shown below) undergoes reduction in the presence of H2 and Pd, two compounds are generated. What is the relationship between the two compounds generated? ОН Но— Н H. OH H OH HO.arrow_forwardCan i get help with this problem?arrow_forwardClassify the following disaccharide. a. beta 1-2 disaccharide b. alpha 1-4 disaccharide c. alpha 1-6 disaccharide d. alpha 1-2 disaccharide e. beta 1-4 disaccharide f. beta 1-6 disaccharidearrow_forward
- The following Statin is called Pravastatin. It is used to help lower high levels of LDL-C in patients with Familial Hypercholesterolemia (FH). USE DIFFERENT COLORS FOR FOLLOWING QUESTIONS! Circle the Pharmacophore. Box the Functional groups.arrow_forwardח' x C A mixed triacylglycerol that contains one saturated 8-carbon fatty acid chain and two 6-carbon fatty acid chains with 2 trans double bonds each.arrow_forwardIntermolecular Forces and Solubility: (Like dissolve like) 1. Which of the following molecules are polar? Label them. 2. Which of the following molecules are nonpolar. Label them. 3. Which of the following compounds are ionic? Label them. 4. Which of the compounds below would dissolve best in cyclohexane and which ones will dissolve best in water? Put a W or C next to each indicated structure to indicate their solubility. Do not put W or C next to cyclohexane or water because they are solvents to dissolve other molecules H2 H2C° CH2 H. H2C. .CH2 water H2 cyclohexane K* i. H2 H2 H3C. K* CH3 H2 H2 H3C CH3 potassium sulfate hexane acetone K,SO4arrow_forward
- Please modify this drug (tamoxifen) to make it water soluble and easy to be absorbed in stomach? H₂C-N CH3 CH3arrow_forward3. A(n) is a molecule that is synthesized in one part of the body and has an effect in a different part. A) fatty acid B) enzyme C) hormone D) vitamin 4. DNA is a double-stranded nucleic acid that exists as a double helix. Which is responsible for holding the double helix of DNA together? A) Phosphodiester bonds between complementary base pairs on each strand B) B-N-glycosidic linkages between the sugar-phosphate backbone of each strand C) Disulfide bonds between the cysteine residues on each strand D) Hydrogen bonding between complementary bases on each strand 5. Which type of inhibitor is most likely to bind somewhere other than an enzyme's active site? A) competitive B) lock and key C) noncompetitive D) substrate 6. The compound shown below is best classified as what type of lipid? HO, A) Triacylglycerol B) Soap C) Fatty acid D) Waxarrow_forward1. Consider the following molecules. Clearly circle the glycosidic bond on each structure. In the Spae provided clearly describe the type of glycosidic bond illustrated. HO-CH2 O. H. H. OH HO. HO-CH2 OH H. H. H. OH H. OH Но H. H. H. OH OH HOH2Ç HO OH OH CH2OH HI エー エエーarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning