
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
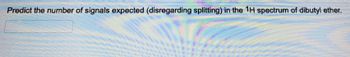
Transcribed Image Text:Predict the number of signals expected (disregarding splitting) in the 1H spectrum of dibutyl ether.
13700
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw here the structure of 2-Chloropropene and encircle or point using arrows the different kinds of protons that would show in its 1H NMR spectrum.arrow_forwardSplitting of a signal in a proton NMR spectrum tells us the number of chemically non-equivalent hydrogens in the immediate vicinity of the hydrogen giving the signal. Predict the number of lines exhibited by hydrogens at the labeled positions in a first-order NMR spectrum. (Make the approximation that all coupling constants are equal.) 2) он The number of lines exhibited by hydrogen(s) a is The number of lines exhibited by hydrogen(s) b is The number of lines exhibited by hydrogen(s) c isarrow_forward11-Explain why 2-chloropropene shows signals for three kinds of protons in its 1H NMRspectrum? Draw the structure and predict the approximate values for 1H-NMR spectra.arrow_forward
- Determining the Molecular Ions for an Alkyl Chloride What molecular ions will be present in a mass spectrum of 2-chloropropane, (CH3)2CHCl?arrow_forwardThe following are esters derived from acetic acid, each with the formula C5H10O2. Label the peaks to indicate the degree of splitting. Use the integral curve traced on the spectrum to calculate the number of hydrogens for each signal and then determine the structure of the ester.arrow_forwardCalculate the exact resonance frequency for the two signals of 1,3-dichloropropane B = 3.75 Cl-C- C-C- B JAB=6.2 Hz A = 2.20 TMS 90 Hz, ア 6 5 4 2 1 O ô ppm I-0-IM I-U-IA I-y-IBarrow_forward
- The mass spectrum of 2,2-dimethylpropane shows only a very weak molecular ion peak at m/z = 72. However, a large peak at m/z = 57 is seen. Suggest a possible structure of the fragment giving rise to this large peak and suggest a reason as to why this peak is so large.arrow_forwardExplain the appearance of the 1H-NMR spectrum of 1,1,2-trichloroethane. How many signals would you expect, and into how many peaks will each of the signals be split?arrow_forwardch1arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY