
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
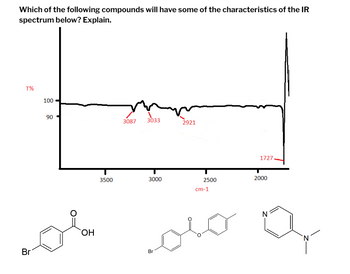
Transcribed Image Text:**Infrared (IR) Spectrum Analysis**
**Which of the following compounds will have some of the characteristics of the IR spectrum below? Explain.**
**IR Spectrum Details:**
- **Transmittance (T%):** The y-axis represents transmittance percentages, ranging from 90% to 100%.
- **Wavenumber (cm⁻¹):** The x-axis displays the wavenumber, ranging from approximately 2000 to 3500 cm⁻¹.
**Key Absorption Peaks:**
1. **3087 cm⁻¹:** This peak is characteristic of C-H stretching in aromatic compounds.
2. **3033 cm⁻¹:** Another C-H stretching peak, typically seen in aromatic rings.
3. **2921 cm⁻¹:** C-H stretching in aliphatic compounds.
4. **1727 cm⁻¹:** A strong absorption band indicative of a C=O stretching vibration, commonly found in carbonyl groups.
**Molecular Structures:**
1. **First Structure:** A brominated aromatic carboxylic acid.
2. **Second Structure:** A brominated aromatic ester.
3. **Third Structure:** A nitrogen-containing heterocyclic compound.
**Analysis:**
- The strong absorption at 1727 cm⁻¹ suggests the presence of a carbonyl group (C=O stretching), which is consistent with the first and second structures (carboxylic acid and ester).
- The peaks around 3087 cm⁻¹ and 3033 cm⁻¹ indicate C-H stretching in aromatic compounds, present in all three structures.
- The peak at 2921 cm⁻¹ reflects aliphatic C-H stretching, which may appear in the ester and the nitrogen-containing heterocycle.
**Conclusion:**
The IR spectrum provided matches the characteristics of compounds with aromatic rings, aliphatic C-H bonds, and a distinct carbonyl group. Both the first and second structures (the brominated aromatic carboxylic acid and ester) closely reflect these features.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the wavenumber of IR radiation designated by 26.44 microns? (Enter your answer as a number. Don't add any symbols or words to your answer.) Hint: One micron is 1.0E-06 meters and one centimeter is 1.0E-02 meters. One wavenumber is one reciprocal centimeter.arrow_forwardQ10) Describe spectrophotometry tests? If you were given a solution that contains an absorbing molecule how would you determine its max absorbance?arrow_forwardCompound C6H10O has the infrared spectrum shown below. Draw areasonable structure that is consistent with this data. Please explain work. Calculate the Rvalue first! -This compound has two common functional groups.arrow_forward
- Pictured is an unknown IR spectrum please include any information you can as to what the identity of the substance is,arrow_forward2. Calculate the absorbance value (A) for a solution that absorbs 89.5% of the light striking it. (Hint: What is the percent transmittance? Review the background reading)arrow_forwardWhich compound matches this IR spectrum?arrow_forward
- Interpret as many of the main peaks in the following spectra as you can... .... .... ...arrow_forward* Question Completion Status: QUESTION 1 What is the sample absorbance of a solution having a percent transmittance of 84.2%? O a. 1.93 O b.0.801 O c. 0.0747 O d.0.158 Click Save and Submit to save and submit. Click Save All Answers to save all answers. search 21arrow_forwardChoose the molecule to which each of these two IR spectrums correspond (one possible answer per IR spectrum).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning