
Introductory Chemistry For Today
8th Edition
ISBN: 9781285644561
Author: Seager
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
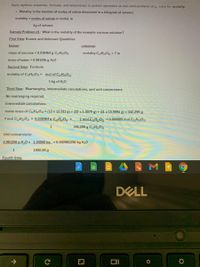
Transcribed Image Text:Apply algebraic properties, formulas, and relationships to perform operations on real-world problems (e.g., solve for: molality.
• Molality is the number of moles of solute dissolved in a kilogram of solvent.
molality = moles of solute or molal, m
kg of solvent
Sample Problem #1: What is the molality of the example sucrose solution?
First Step: Known and Unknown Quantities
known
unknown
mass of sucrose = 0.030464 g C12H2201
molality C12H2201 = ? m
mass of water = 0.981256 g H,0
Second Step: Formula
molality of C12H22O11 = mol of C12H22O1
1 kg of H,0
Third Step: Rearranging, intermediate calculations, and unit conversions
No rearranging required.
Intermediate calculations:
molar mass of C12H2201 = (12 x 12.011 g) + (22 x 1.0079 g) + (11 x 15.9994 g) = 342.299 g
# mol C12H2201 = 0.030464 g C12H,O1 x
1 mol C,,H„01 = 0.000089 mol C12H22O1
1
342.299 g C12H2201
Unit conversions:
0.981256 g H,Ox_1.00000 kg_ = 0.000981256 kg H,0
1
1000.00 g
Fourth Step:
DELL
「田
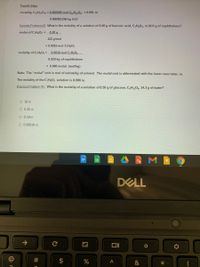
Transcribed Image Text:Fourth Step:
molality, C12H2201 = 0.000089 mol C1,HO1 = 0.091 m
0.000981256 kg H20
Sample Problem #2: What is the molality of a solution of 0.20 g of benzoic acid, C,H,O2, in 20.0 g of naphthalene?
moles of C,HO2 =
0.20 g
122 g/mol
= 0.0016 mol C,H¢O2
molality of C,HO2 =
0.0016 mol C,H;O>
0.020 kg of naphthalene
= 0.080 molal (mol/kg)
Note: The "molal" unit is mol of solute/kg of solvent. The molal unit is abbreviated with the lower case letter, m.
The molality of the C;HgO2 solution is 0.080 m.
Practice Problem #1: What is the molality of a solution of 0.50 g of glucose, CH12O6, 14.3 g of water?
O 35 m
O 0.35 m
O 0.19 m
O 0.00019 m
DELL
&
司
%24
#3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Match the vapor pressure diagrams with the solute-solvent combinations and explain your answers. a. and b. and c. and d. andarrow_forwardThe Henry's law constant for the solubility of radon in water at is 9.57106 M/mm Hg. Radon is present with other gases in a sample taken from an aquifer at 30C. Radon has a mole fraction of 2.7106 in the gaseous mixture. The gaseous mixture is shaken with water at a total pressure of 28 atm. Calculate the concentration of radon in the water. Express your answers using the following concentration units. (a) molarity (b) ppm (Assume that the water sample has a density of 1.00 g/mL.)arrow_forwardA patient has a “cholesterol count” of 214. Like manyblood-chemistry measurements,this result is measured inunits of milligrams per deciliter (mgdL1). Determine the molar concentration of cholesterol inthis patient’s blood, taking the molar mass of cholesterolto be 386.64gmol1. Estimate the molality of cholesterol in the patient’sblood. If 214 is a typical cholesterol reading among men inthe United States, determine the volume of such bloodrequired to furnish 8.10 g of cholesterol.arrow_forward
- Mixtures of gases are always true solutions. True or false? Explain why.arrow_forwardYou have read that adding a solute to a solvent can both increase the boiling point and decrease the freezing point. A friend of yours explains it to you like this: The solute and solvent can be like salt in water. The salt gets in the way of freezing in that it blocks the water molecules from joining together. The salt acts like a strong bond holding the water molecules together so that it is harder to boil. What do you say to your friend?arrow_forwardA certain gaseous solute dissolves in water, evolving 12.0 kJ of heat. Its solubility at 25C and 4.00 atm is 0.0200 M. Would you expect the solubility to be greater or less than 0.0200 M at (a) 5C and 6 atm? (b) 50C and 2 atm? (b) 20C and 4 atm? (b) 25C and 1 atm?arrow_forward
- Infer Dehydration occurs when more fluid is lost from the body than is taken in. Scuba divers are advised to hydrate their bodies before diving. Use your knowledge of the relationship between pressure and gas solubility to explain the importance of hydration prior to a dive.arrow_forwardHow do colloids differ from solutions with regard to dispersed particle size and homogeneity?arrow_forwardCaffeine is made up of 49.5% C, 5.2% H, 16.5% O, and 28.9% N. A solution made up of 8.25 g of caffeine and 100.0 mL of benzene (d=0.877g/mL) freezes at 3.03C. Pure benzene (k f =5.10C/m) freezes at 5.500C. What are the simplest and molecular formulas for caffeine?arrow_forward
- 6-21 Are mixtures of gases true solutions or heterogeneous mixtures? Explain.arrow_forwardAntifreeze solutions are aqueous solutions of ethylene glycol, C2H6O2(d=1.12g/mL). In Connecticut, cars are winterized by filling radiators with an antifreeze solution that will protect the engine for temperatures as low as -20F. (a) What is the minimum molality of antifreeze solution required? (b) How many milliliters of ethylene glycol need to be added to 250 mL of water to prepare the solution called for in (a)?arrow_forwardHow do solutions differ from compounds? From other mixtures?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning