
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
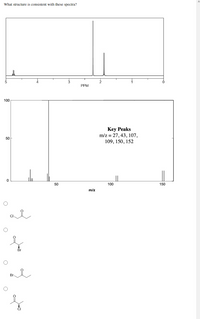
Transcribed Image Text:What structure is consistent with these spectra?
PPM
100
Кey Peaks
m/z = 27, 43, 107,
50
109, 150, 152
50
100
150
m/z
al
Br-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Fill out the IR spectra of the compoundarrow_forward100 C4H200 O C3H₁3Cl O C5H10N C6H14 C6H12 80 60 40 20 0 10 20 40 60 m/z 80 100 120 140 MassSpec9. Select the most reasonable formula for the molecule analyzed, based on the provided data (parent peak m/z at 84):arrow_forwardIR Spectrum (liquid film) 184 4000 100 80 60 40 20 40 13C NMR Spectrum (50.0 MHz, CDCI, solution) proton decoupled 200 ¹H NMR Spectrum (600 MHz, CDCI, solution) 1 10 9 8 3000 % of base peak 80 128 my 2000 V (cm¹) 119 M+* 134 120 160 m/e expansion 124 ppm 160 1600 I 7 200 I 6 120 ppm 1200 800 Mass Spectrum C10 H14 280 solvent 80 240 I 5 7.4 1 4 expansion 3 Problem 95 UV Spectrum max 262 nm (log10 € 2.5) solvent: ethanol -CH3 C 40 7.2 2 1 0 8 (ppm) TMS 1 I 1 0 8 (ppm) 201arrow_forward
- Which C-H bonds are responsible for the following peaks/signals? C6H1202 s are Peak A = Peak B= Peak C= Enter 12 if a H is not labeled that created the peak/signal. this peak disappears with the addition of D20 By PPMarrow_forwardFind the Mass Specarrow_forwardWrite the condensed structural formula for the following molecule Y For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac). BIUS Paragraph V V Arial 10ptarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY