
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please what molecule is this
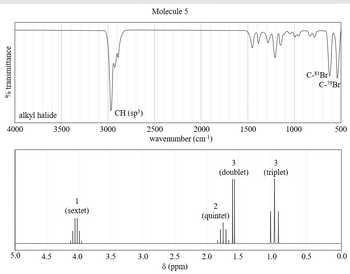
Transcribed Image Text:The image consists of two graphs which provide spectroscopic data for "Molecule 5."
### Top Graph: Infrared (IR) Spectrum
- **Y-axis:** % Transmittance
- **X-axis:** Wavenumber (cm⁻¹), ranging from 4000 to 500
#### Key Features:
- **Alkyl Halide Absorption:** Noted around the 3000 cm⁻¹ mark, indicating the presence of C-H (sp³) bonds.
- **Significant Peaks:** Two peaks are labeled as C-81Br and C-79Br, appearing close to 500 cm⁻¹, indicative of C-Br bond stretching.
### Bottom Graph: Nuclear Magnetic Resonance (NMR) Spectrum
- **Y-axis:** No specific label, generally represents intensity.
- **X-axis:** Chemical shift (δ, ppm), ranging from 5.0 to 0.0
#### Peak Characteristics:
1. **1 (sextet):** Appears around 3.8 ppm, likely representing a split peak due to coupling with adjacent protons.
2. **2 (quintet):** At approximately 1.7 ppm, showing a quintet pattern.
3. **3 (doublet and triplet):**
- *Doublet:* Around 1.4 ppm, typically suggesting two neighboring equivalent hydrogens.
- *Triplet:* Near 1.0 ppm, indicating three neighboring equivalent hydrogens.
These spectra are typically used to identify functional groups and structural components within the molecule, aiding in molecular characterization and analysis.
Expert Solution
arrow_forward
Step 1: Introduce question
The question is based on the concept of organic spectroscopy. We need to analyse the spectral data and identify the compound.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the iupac name of this moleculearrow_forwardName this molecule using the systematic nomenclature rules used in the textbook and in class. Use all lower case and enter the name as one word, leaving no spaces. m CI CIarrow_forwardWhich would be correct please? do I separate the OH?arrow_forward
- what functional groups are shown herearrow_forwardFor the following molecule, give, according to IUPAC standards, it's name. Follow the rules for commas, spaces and dashes.arrow_forwardI am studying for an o-chem exam and practicing name structures but there isn't an answer key. What is the correct name of these structures?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY