
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please provide the reagents for the following transformations.
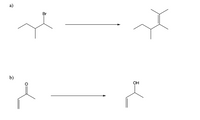
Transcribed Image Text:а)
Br
b)
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- CH3NH2 is a (a)Arrhenius acid. (b)Arrhenius base. (c)Bronsted-Lowry acid. (c)Bronsted-Lowry base. (e)Louis base.arrow_forwardFor the following acid-base reaction, (1) predict the products, showing both reactants and products complete Lewis structures and arrows showing electron flow; (2) label each structure with the lowing: Bronsted acid, Bronsted base, conjugate acid, conjugate base; (3) give a brief definition of a ronsted acid and Bronsted base; (4) predict the direction of the equilibrium and justify your answer. HC0OH + CH3 Nta PRん106Y pkb = 3.36arrow_forwardHCl (excess) H₂O₂arrow_forward
- 2-11 2.19] A solution has an H+ concentration of 10-5 M. (a) What is the pH of this solution? (b) What is the pOH (Assume that the temperature of the solution is 25°C.) Answer: (a) 5 (b) 9 BMarrow_forwardThe pH scale for acidity is defined by pH – log10 H*| where |H*| is the concentration of hydrogen ions measured in moles per liter (M). (A) The pH of Drano is 13.3. Calculate the concentration of hydrogen ions in moles per liter (M). [H*] = M (B) The pH of rain water is 5.5. Calculate the concentration of hydrogen ions in moles per liter (M). [H*] = Marrow_forwardWhy is (CH3)3Cl considered to be a Lewis Acid?arrow_forward
- CO2 + H2O→H2CO3 → HCO3– +H+ → CO2– +2H This chemical reaction can produce 1) a small number of hydrogen ions 2) a large number of hydrogen ions 3) a high pH value 4) anoxiaarrow_forwardCalculate the equilibrium constant for the acid–base reaction between the reactants in each of the following pairs: (a) HCl + H2O (b) CH3COOH + H2O (c) CH3NH2 + H2O (d) CH3N+H3 + H2Oarrow_forward(h) (i) (i) NH₂ (1) (CH3)2NH CHOH acetic acid (1) NaH (2) AIC13 (1) NaH OF (2) Br H Br NMe₂ (1) POC13 (2) Na₂CO3, H₂Oarrow_forward
- The pH reading of a sample of each substance is given. Calculate the hydrogen ion concentration of the substance. (Give your answers in scientific notation, correct to one decimal place.) (a) Spinach: pH = 5.4 [H+] = 3.98107 -6 x 10 M (b) Crackers: pH = 7.2 [H*] = х 10 Marrow_forwardThe amount of tartaric acid is responsible for the tartness of wine and controls the acidity of the wine. Tartaric acid also plays a very significant role in the overall taste, feel and color of a wine. Tartaric acid is a diprotic organic acid The chemical formula for tartaric acid is C4H6O6 and its structural formula is HO2CCH(OH)CH(OH)CO2H. A 50.00 mL sample of a white dinner wine required 21.48 mL of 0.03776 M NaOH to achieve a faint pink color. Express the acidity of the wine in terms of grams of tartaric acid, H2C4H4O6 (M. M. = 150.10) per 100 mL of wine. Assume that the two acidic hydrogens are titrated at the end point. MM H2C4H4O6 = 150.10 MM NaOH = 40.00 Below is the balanced chemical equation for this titration.arrow_forwardPredict whether aqueous solutions of the following substances are acidic, basic, or neutral and write hydrolysis equations for the acidic and basic solutions. (a) CsBr; (b) Al(NO3)3; (c) KCN; (d) CH3NH3Clarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning