
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Nitesh
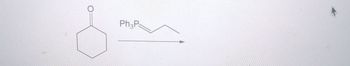
Transcribed Image Text:Ph3P
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Alliin C6H11NO3S how many grams of sulfur in 0.358730606mol show work/ equationsarrow_forwardConversion 95 mol H2O to kg H2Oarrow_forwardIf the Day 2 filter paper was not left in the drying oven for long enough (i.e. the time specified in the lab manual was long enough), it would still be wet. How would this affect your calculated percent yield. What type of error is this? decreased percent yield; systematic error decreased percent yield; random error increased percent yield; random errorincreased percent yield; systematic errorarrow_forward
- please help find the Percent yeild and limiting regent given the collected data Volume acetic acid: 20.0 mLVolume Isopentyl alcohol: 15.0 mLMass of Isopentyl Acetate collected: 9.775 gramsDensity of acetic acid: 1.049 g/mLDensity of Isopentyl alcohol: 0.813 g/mL i mostly need help with the yieldarrow_forwardwhat is the meaning of dissociation?arrow_forwardThe illustration to the left represents a mixture of hydrogen ( light blue ) and iodine ( purple ) reacting to form a product. In the illustration: The product that forms is eq The number of product molecules formed is теq The limiting reagent is теq The number of atoms/molecules in excess is req Previous a $ 4, hp ins prt sc delete home f10 f11 f12 トト」 &a backspace unu lock L. 0O enter HS pause 00arrow_forward
- Given the unbalanced equation: Al + CuO Al2O3 + Cu. When properly balanced, the balancing coefficient of Al2O3 is...? please explain completelyarrow_forwardQUESTION 7 Waking fast can consume 50keal per minute Hor many hours of eercbe are required to consume 450 kcal, the energy in a large candy bar? OA 125 O8175he OC75 OD15 OE1 QUESTION 8 Which of the lellwing does not have a unom composition troughout? OA Compound OB Heterogeneous mature OC Homogeneous midure OD Element OE Solvent Click Save ond Sumit to save and submit. Click Sene All Ansers to pae dil anrsarrow_forwardNitroglycerin (C3H5N3O9) is a powerful explosive. Its decomposition may be represented by 4C3H5N3O9 ⟶ 6N2 + 12CO2 + 10H2O + O2 This reaction generates a large amount of heat and many gaseous products. It is the sudden formation of these gases, together with their rapid expansion, that produces the explosion. (a) What is the maxi- mum amount of O2 in grams that can be obtained from 2.00 × 102 g of nitroglycerin? (b) Calculate the percent yield in this reaction if the amount of O2 generated is found to be 6.55 g.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY