
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
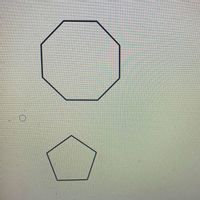
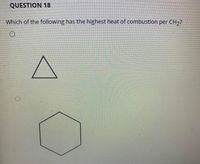
Transcribed Image Text:QUESTION 18
Which of the following has the highest heat of combustion per CH,?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which state has more Kinetic energy?a) Liquid Water b) Solid water c) Water vaporarrow_forwardQuestion 10 When the water formed as theresult of combustion is condensed to a liquid product, the resulting latent-heat release the heat given off as a result of the combustion reaction A subtracts (B) adds doubles (D equalsarrow_forward(Mass of crucible and cover is 43.92 g. The mass of crucible, cover, and magnesium is 44.62g. Mass of crucible, cover, and magnesium oxide is 44.99g) a) Mass of Mg reacted b) Mass of magnesium oxide formed c) Mass of oxygen in magnesium oxidearrow_forward
- What is the enthalpy change when 49.4mL of .430 M sulfuric acid reacts with 23.3 mL of .309 M potassium hydroxidearrow_forwardA hydrated barium hydroxide compound is heated to remove all the water of hydration. The mass of the compound before heating was 15.6 g and after heating the mass was recorded as 8.75 g. a) Determine the chemical formula of the hydrate. b) The chemist who performed the experiment was concerned about how long to heat the hydrated crystal. The compound is known to be barium hydroxide octahydrate. In the experiment above, is it more likely that the hydrate was heated slightly too long (or perhaps at too high a heat) or not long enough? Explain your choice.arrow_forwardGasoline is composed of a variety of different liquid hydrocarbons, which do not separate as time passes. Gasoline is an example of a: A) heterogeneous mixture B) Chemical compound C) Chemical element D) Solutionarrow_forward
- What are other examples of combustion in the human body?arrow_forwardQuestion 3 Bethany needs to measure out a certain amount of Cuso, for a lab experiment. The proced- ure only gives her the amount in moles. What should she use to calculate the amount of grams of Cuso. needed?arrow_forwardName (please print): Heat Capacity of an Unknown Metal Post Lab Questions 1.) When determining the heat capacity of the unknown metal we assumed the heat capacity of the water has a value of 4.186J/g®C. Is there an error(s) in making this error? Explain The possibility of error in the experiment can be loss of heat, the thermometer not being precise enough to give accurate values, and the capacity of the thermometer is being ignored 2.) What is the heat energy needed to raise the temperature of 6.63moles of ethanol CH;CH;OH from a temperature of 2.33°C to 17,5°C. [CH;CH;OH=46.07g/mol) [Ccusch2ow-2,46J/g°C] 3.) What is the identity of an unknown metal that absorbs 0.589KJ of heat energy where the metal is heated from 33.6°C to 241.6°C. The mass of the metal is 13.48g. [Cr= 0.160 J/g°C] [Cs»= 0.210 J/g°C] [Cc. = 0.385 J/g°C][Cz» = 0_390 J/g*C] 4.) How much encrgy is liberated when 600.2g of O, is reacted with propane in a combustion tank. Assume the following: C,Hg) + 50;(g) – 3C0;(g)+…arrow_forward
- What general form of energy is initially in your gas tank? What form of energy is it converted to when the car is moving?arrow_forwardConsidering the amount of energy absorbed or released, what type of reaction occurs when gasoline is consumed in a car?arrow_forwardAcetylene (C,H,) gas is often used in welding torches because of the very high heat produced when it reacts with oxygen (O. gas, producing carbon dioxide gas and water vapor. Calculate the moles of acetylene needed to produce 1.50 mol of carbon dioxide. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits. olo &arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY