
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
I want to complete the schedule on all existing
vehicles
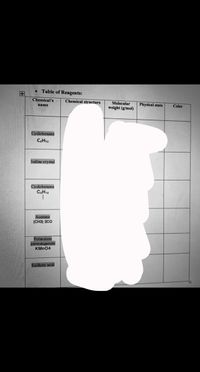
Transcribed Image Text:• Table of Reagents:
田
Chemical's
Chemical structure
Molecular
weight (g/mol)
Physical state
Color
name
Cydohexane
CH12
Iodine crystal
Cydohexene
C.H10
Acetone
(CH3) 2CO
Potas sium
parmanganate
KMN04
Sulfuric acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Dilution is an irreversible process. True Falsearrow_forward6.A fuel is burned with 50% excess air and the combustion characteristics of the fuel oil are similar to C12H26. Determine the ff. a) air fuel ratio b) volumetric (molal) analysis of the products of combustion.arrow_forwardTime(s) [X3] (mole/L) 0 0.600 200 0.458 400 0.362 600 0.281 800 0.204 1000 0.175 1200 0.134 1400 0.104 1600 0.083 1800 0.063 2000 0.047 plot ln[X3] vs time plot 1/[X3] vs timearrow_forward
- O Course Home b Details | bartleby O X -> A openvellum.ecollege.com/course.html?courseld=16561285&OpenVellumHMAC=dd25775cc7b85cec3204f753df519590#10001 Q * E Apps O Maps E Connect - To Do As... O ocCC Moodle P chem work b help A Balance Chemical E. Gmail YouTube CHEM-1123-EW40S Late Spring 2021 Sign Out Help Hi, ashlee v Mastering Chemistry Course Home O My Courses Syllabus Part A Scores Identify which of the following are carbohydrates. Check all that apply. еТext • View Available Hint(s) Document Sharing Но User Settings CH, Course Tools > H CH-O, H OH H C-C OH H OH OH H H O H-Ö -NH, H. ОН ОН ОН H H H H-C- -C С—С—ОН ОН ОН ОНн O H O HO-C- -C-C-OH ОН ОН ОН 10:39 PM P Type here to search 99 3/10/2021arrow_forwardA chemist must dilute 0.0757 L of 11.6 M aqueous silver nitrate (AgNO,) solution until the concentration falls to 5.00. M . She'll do this by adding distilled water to the solution until it reaches a certain final volume. Calculate this final volume, in liters. Round your answer to 3 significant digits. x10 Continue Submit Assignr 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Access IMG-4474.jpg IMG-4472.jpg IMG-4471.jpg IMG-4467.jpg IMG-4464.jpg MacBook Air esc 80 DII DD F1 F2 F3 F4 F6 F5 F7 F8 F9 F10 F11 @ # % & ( ) 1 2 3 4 5 6 7 8 9arrow_forward1. A gas consist of 70% propane and 30% butane by volume. Find the stoichiometric air-to-fuel ratio. a. 3:14 b. 14: 3 c. 23:1 d. 1:23 2. A furnace is fired with fuel oil with a partial analysis of 7.6% S and 2.8% N. Orsat analysis of the stack gas shows 9.44% CO2, 1.19% CO, 0.4% SO2, 0.47% H2, 6.8% O2 and 81.7% N2. Air supplied is at 23 deg Celsius, 755 mmHg and 85% RH. Calculate the percent excess air. a. 55% b. 78% c. 23% d. 38%arrow_forward
- 5. Generally, in order to do a stoichiometry problem, you need a complete balanced equation. However, in some cases it is possible to do some stoichiometric calculations without the complete equation as long as a particular element is present in only one reactant and one product. For example, if you were told that an experiment converted FeCl3 to Fe3O4 and that those were the only reactants and products that contained iron (Fe), you would know that there would have to be 3 moles of FeCl3 for every 1 mole of Fe;O4 or else the iron would not balance. You would not know for sure whether the balancing coefficients were actually 3 and 1 (They might be 6 and 2, 9 and 3, 12 and 4, etc) but you would know that they had to be in a 3 to 1 ratio and that would be enough to relate those two compounds with the correct stoichiometry. a) In Part I of your experiment, potassium carbonate (K,CO3) was the only reactant which contained carbon (C) and strontium carbonate (SrCO3) was the only product…arrow_forwardcollege.com/course.html?courseld3 154322748HepID=2b3e48e6520860bfd5591538a4a5a27b#10001 Search... y alosles are o USAonline: IMS 460-801F2 ay ceTripAdvisor (MC 13 on Temperature, Changes in Energy, and Gases Item 22 A Review | Constants Use molar volume to solve the following problems at STP. Part C Ne Find the number of grams of neon contained in 11.4 L gas. AZ4 Request Answer Submit Part D Find the number of moles of H, in 1820 mL H, gas. ΗΑΣ. P Pearson GLAYA Contact Us Permissions Privacy Policy Terms of Use Copyright © 2019 Pearson Education Inc. All rights reserved. hp hp dts Studio Sound f12 f1o f9 f8 f7 +1 f6 fs f4 & 23 3. 6. 8. 7. 6. 4. P. Y. R %24arrow_forwardHg²+ + 2 e → Hg Hg2* + 2 e → 2 Hg Hg,Br2 + 2 e –→ 2 Hg + 2 Br MnO4 + 4 H* + 3 e¯ → MNO2 + 2 H2O E° = +1.68 V Pb + 2 e –→ Pb Sn²* + 2 e → Sn E° = +0.851 V 2+ E° = +0.797 V E° = -0.126 V E° = +0.139 V E° = –0.02 V Calculate the solubility product for Hg2Br2.arrow_forward
- Data Set 1 Mass of Mg metal: 0.0462g Temperature of the water in the beaker: 22.0°C Total Volume of Gas Collected: 44.1 mL Barometric Pressure: 753 torr. Height of water column in Eudiometer: 0.0 mm Data Set 2 Mass of Mg metal: 0.0441 g Temperature of the water in the beaker: 22..6°C Total Volume of Gas Collected: 43.6 mL Barometric Pressure: 753 torr. Height of water column in the eudiometer: 19.0 mmarrow_forwardWhat's a single displacementarrow_forwardJust #2 pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY