
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
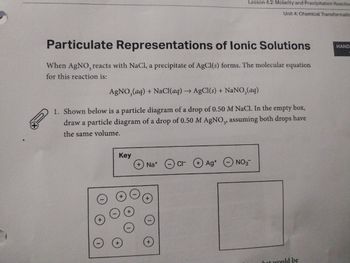
Transcribed Image Text:Particulate Representations of lonic Solutions
When AgNO, reacts with NaCl, a precipitate of AgCl(s) forms. The molecular equation
for this reaction is:
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
1. Shown below is a particle diagram of a drop of 0.50 M NaCl. In the empty box,
draw a particle diagram of a drop of 0.50 M AgNO,, assuming both drops have
the same volume.
Key
Lesson 4.2: Molarity and Precipitation Reaction
Unit 4: Chemical Transformatic
Na+ cr
Ag+
NO3
hat would be
HAND
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the ionic equation and the net ionic equation for this reaction. Include all the physical states, and circle the spectator ions in the complete ionic equations.arrow_forwardGive the complete ionic and net ionic equation for the reaction below. Circle the spectator ions. Fe(s) + 2 AgNO3(aq) → 2 Ag(s) + Fe(NO3)2(aq)arrow_forwardFor the reaction Ba(OH)2 (aq) + (NH4)½CO3 (aq)--> What one of the following species would appear in the molecular equation? (Don't worry about coefficients. Just write the molecular equation, and see which of these appears in it.) OH (s) Ba2 (aq) O NH,OH (s) BaCO3 (s) O NH2"(aq)arrow_forward
- The maximum contaminant level of cyanide (CN) in drinking water as set by the the Environmental Protection Agency (EPA) is 0.00020 g · L. Express this concentration in parts per million (ppm). Assume the density of water is 1.00 g/mL. concentration: Ppmarrow_forwardConsider the following chemical reaction. 3 Zn(C₂H₃O₂)₂(aq) + 2 Na₃PO₄(aq) → 6 NaC₂H₃O₂(aq) + Zn₃(PO₄)₂(s) A chemist makes a solution of zinc acetate by dissolving 20.5 g Zn(C₂H₃O₂)₂ in 105 mL H₂O. What is the concentration of this zinc acetate solution?arrow_forwardInsoluble PbBr2(s) precipitates when solutions of Pb(NO3)2 and NaBr are mixed. Pb(NO3)2(aq) + 2 NaBr(aq) ----------> PbBr2(s) + 2 NaNO3(aq) 200.0 mL of 0.750 M Pb(NO3)2 and 200.0 mL of 1.50 M NaBr are mixed together in a coffee cup calorimeter. The temperature of the mixture rises by 2.44°C. Calculate whether a limiting reactant is present or if perfect amounts of both reactants are present. Calculate the enthalpy change for this precipitation reaction. (assume the density of all solutions is 1.00 g/mL). (Answer in kJ)arrow_forward
- If 785 mL of 0.350 M AgNO₃ is added to 475 mL of 0.250 M CaBr₂, how many moles of AgBr precipitate will be formed? The balanced equation is 2 AgNO₃(aq) + CaBr₂(aq) → 2 AgBr(s) + Ca(NO₃)₂(aq)arrow_forwardPotassium iodide reacts with lead(II)(II) nitrate in the following precipitation reaction: 2KI(aq)+Pb(NO3)2(aq)→2KNO3(aq)+PbI2(s)2KI(aq)+Pb(NO3)2(aq)→2KNO3(aq)+PbI2(s) What minimum volume of 0.218 MM potassium iodide solution is required to completely precipitate all of the lead in 145.0 mLmL of a 0.150 MM lead(II)(II) nitrate solution?arrow_forwardFind the volume of 0.339 M HCl(aq) required to completely neutralize 0.278 g of Al(OH)3(s) according to the unbalanced reaction: HCl(aq) + Al(OH)3(s) → AlCl3(aq) + H2O(l) The molar mass of Al(OH)3 is 78.0 g/mol. Give the answer in mL with 3 or more significant figures.arrow_forward
- You titrate a 25.00 mL solution of hydrochloric acid, HCI, with a 0.2015 M sodium hydroxide, NaOH, solution. The titration required 39.73 mL of the sodium hydroxide to reach the endpoint. What is the concentration (molarity) of the hydrochloric acid? HCI + NaOH → NaCl + H₂O Student work: 39.73 mL NaOH 1 L 1000 ML 25.00 mL HCI 1000 mL HCI 1 L HCI 0.0080055 mol HCI 0.02500 L HCI 0.2015 mol NaOH 1 L NaOH 0.02500 mol 0.3202 M HCl 0.0080055 mol Read the feedback statements below and chose all that are applicable to the work shown. Choose one or more: The unit cancellation is not correct. The conversions to from mL to L do not need to be shown since they cancel out. O The final answer has an incorrect number of s.f. (it should have 4 s.f.) U The final answer has an incorrect number of s.f. (it should have 3 s.f.) [] Units do not cancel properly. The stoichiometry step to convert mol NaOH to mol HCI is not shown. U All data collected, work shown, and final answer are recorded and shown…arrow_forwardWhile working in a lab, Ronan reacts 50.0 mL of 0.320 mol/L Na2CO3 (aq) with 85.0 mL of 0.210 mol/L AlCl3 (aq). Calculate the mass of Al2(CO3)3 precipitate that forms when the reaction is complete 3 Na2CO3 (aq) + 2 AlCl3 (aq) → Al2(CO3)3 (↓) + 6 NaCl (aq)arrow_forward4. Calculate the calculate the mass of precipitate formed from 5.0 g of strontium chloride. Li3PO4(aq) → Sr3(PO4)2(S) + LiCl(aq) _SrCl₂(aq) + 5. What mass of aluminum hydroxide in needed to neutralize 5.00 g of hydrochloric acid? Al(OH)3(s) + _HCl(aq) → ___AlCl3(aq) + _H₂O(1) 195arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY