
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
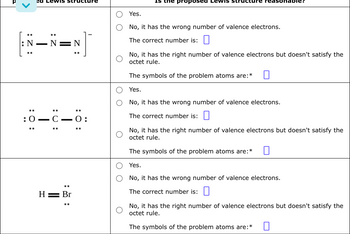
Transcribed Image Text:<
:
ed Lewis structure
N
0_C_0:
H = Br
O:
the proposed Lewis str
Yes.
No, it has the wrong number of valence electrons.
The correct number is:
No, it has the right number of valence electrons but doesn't satisfy the
octet rule.
The symbols of the problem atoms are: *
Yes.
No, it has the wrong number of valence electrons.
The correct number is:
No, it has the right number of valence electrons but doesn't satisfy the
octet rule.
The symbols of the problem atoms are:*
Yes.
No, it has the wrong number of valence electrons.
The correct number is:
No, it has the right number of valence electrons but doesn't satisfy the
octet rule.
The symbols of the problem atoms are:*
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- P ed Lewis structure Cl =0. :0 :Z: C=N :C=0: Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:*arrow_forward1-BeCl2 2-CH4 3-SF6arrow_forwardWhich atom, if any, in the shown covalent bonds acquires a partial NEGATIVE charge? [References) Specify the correct atom by its chemical symbol, e.g. Br. If none of them bears a negative charge, write, "none." (To answer this question you may consult the table of electronegativity values in the table below.) H. 2.1 1A 2A 3A 4A 5A 6A 7A Li 1.0 Be 1.5 2.0 2.5 3.0 3.5 4.0 Na Mg 0.9 8B Al Si P. CI 2.5 3.0 1.2 3B 4B 5B 6B 7B 1B 2B 1.5 1.8 2.1 K 0.8 Са 1.0 Sc 1.3 Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge 1.6 1.6 1.8 2.0 2.4 2.8 As Se Br 1.5 1.6 1.6 1.5 1.8 1.8 1.8 1.9 Rb Y 1.0 1.2 Sr Zr 1.4 Nb Mo Te 1.6 1.8 1.9 Ru 2.2 Rh 2.2 Pd Ag Cd In Sn Sb 1.7 1.8 Te 0.8 2.2 1.9 1.7 1.9 2.1 2.5 Au Hg Pt 2.2 TI Pb Cs Ba 0.7 0.9 Bi Po At 1.8 1.9 2.0 2.2 La Та W Re 1.7 1.9 2.2 Os Ir 1.1 1.3 1.5 2.2 2.4 1.9 1.8 1.5-1.9 2.5-2.9 <1.0 1.0-1.4 2.0-2.4 3.0-4.0 (а) C-C1 (b) C-F Farrow_forward
- u have good explanationsarrow_forwardRecognizing exceptions to the octet rule Jacqueline Decide whether the Lewis structure proposed for each molecule is reasonable or not. Is this a reasonable structure? If not, why not? molecule proposed Lewis structure O Yes, it's a reasonable structure. O No, the total number of valence electrons is wrong. IBr2 - I- Br: The correct number is:| O No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are:U O Yes, it's a reasonable structure. O No, the total number of valence electrons is wrong. F- P - F: PF. The correct number is: :F: :F : O No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: O Yes, it's a reasonable structure. O No, the total number of valence electrons is wrong. H-N-H NH, The correct number is:|| No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are:I * If two or more atoms have the wrong number of valence electrons around them, just…arrow_forwardList everything that is wrong with the Lewis Structure shown? H-C=N:arrow_forward
- Lewis Structures Lewis Structures are used to describe the covalent bonding in molecules and ions. Draw a Lewis structure for SF, that obeys the octet rule if possible and answer the following questions based on your drawing. 1. For the central sulfur atom: The number of lone pairs = The number of single bonds = The number of double bonds = 2. The central sulfur atomarrow_forwardproposed Lewis structure Is the proposed Lewis structure reasonable? O Yes. O No, it has the wrong number of valence electrons.. H-C N :F OF: O The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* O Yes. ☐ O No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ O Yes. O No, it has the wrong number of valence electrons. H-CEC-H The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. H –0 The correct number is:|| No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. ä=ö| The correct number is: || : Cl =0: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: N No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "O,0". :Z : :Z :arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY