
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
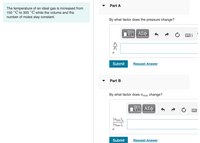
Transcribed Image Text:Part A
The temperature of an ideal gas is increased from
150 °C to 300 °C while the volume and the
number of moles stay constant.
By what factor does the pressure change?
Submit
Request Answer
Part B
By what factor does vrms change?
(Vrms )2
(Vrms )1
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Modern vacuum pumps make it easy to attain pressures of the order of 10-13 atm in the laboratory. For related problem-solving tips and strategies, you may want to view a Video Tutor Solution of Atomic and molecular mass. Part A At a pressure of 6.20x10-14 atm and an ordinary temperature of 296.0 K, how many molecules are present in a volume of 1.08 cm³ ? Enter your answer numerically. N = Submit Part B IVE ΑΣΦ N = Request Answer ? How many molecules would be present at the same temperature but at 1.04 atm instead? Enter your answer numerically. VE ΑΣΦ molecules ? moleculesarrow_forward5.0 g of nitrogen gas at 20°C and an initial pressure of 2.8 atm undergo a constant-pressure expansion until the volume has tripled. Part A What is the gas volume after the expansion? Express your answer with the appropriate units. ► View Available Hint(s) V= Submit HA Value Previous Answers Units ? X Incorrect; Try Again; 5 attempts remainingarrow_forwardR Review I Constants Part A A container holds 1.0 g of argon at a pressure of 8.0 atm. How much heat is required to increase the temperature by 100° C at constant volume? Express your answer with the appropriate units. H HẢ Q = Value Units %3D Submit Request Answer Part B How much will the temperature increase if this amount of heat energy is transferred to the gas at constant pressure? Express your answer in kelvins. ΑΣφ K AT =arrow_forward
- please answer varrow_forwardA 2100 cm³ container holds 0.10 mol of helium gas at 350°C. Part A How much work must be done to compress the gas to 1200 Cm² at constant pressure? Express your answer with the appropriate units. ▸ View Available Hint(s) W = 220 J ✓ Correct Part B Previous Answers How much work must be done to compress the gas to 1200 Cm³ at constant temperature? Express your answer with the appropriate units. ► View Available Hint(s) W = Submit μA Value Previous Answers Units ? X Incorrect; Try Again; 4 attempts remainingarrow_forwardA 22-cm-diameter vertical cylinder is sealed at the top by a frictionless 16 kg piston. The piston is 79 cm above the bottom when the gas temperature is 303° C. The air above the piston is at 1.00 atm pressure. Part A What is the gas pressure inside the cylinder? Express your answer with the appropriate units. ► View Available Hint(s) Po = Submit Part B μÀ Value Units ? What will the height of the piston be if the temperature is lowered to 13°C? Express your answer with the appropriate units.arrow_forward
- For a planet to have an atmosphere, gravity must be sufficient to keep the gas from escaping The escape speed a particle needs to escape the earth's gravitational attraction is 1.1 x 10 m/s. The motion of projectiles never depends on mass, so this escape speed applies equally to rockets and to molecules in the earth's upper atmosphere. Part A At what temperature does the rms speed of nitrogen molecules equal the escape speed? Express your answer in kelvins. ΜΕ ΑΣΦΑ T- Submit Part B T- Request An At what temperature does the mms speed of hydrogen molecules equal the escape speed? Express your answer in kelvins. VAZO Submit ? K Karrow_forwardAn ideal gas at 20°C consists of 2.2 x 1022 atoms. 3.2 J of thermal energy are removed from the gas. Part A What is the new temperature in °C? Express your answer in degrees Celsius. DA ΑΣφ T = °C Submit Request Answerarrow_forwardPart C Some hydrogen gas is enclosed within a chamber being held at 200°C with a volume of 0.0250 m³. The chamber is fitted with a movable piston. Initially, the pressure in the gas is 1.50 × 106 Pa (14.8 atm). The piston is slowly extracted until the pressure in the gas falls to 0.950 x 106 Pa. What is the final volume V₂ of the container? Assume that no gas escapes and that the temperature remains at 200°C. Enter your answer numerically in cubic meters. ► View Available Hint(s) V₂ = Submit [5] ΑΣΦ ? m³arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON