
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
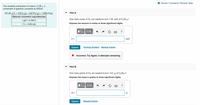
Part A: How many moles of O2 are needed to burn 1.65 mol of C8H18? Express the amount in moles to three significant digits.
Part B:
How many grams of O2 are needed to burn 14.0 g of C8H18?
Express the mass in grams to three significant digits.
Part C:
Octane has a density of 0.692 g/mL at 20∘C. How many grams of O2 are required to burn 14.0 gal of C8H18?
Express the mass in grams to three significant digits.
Transcribed Image Text:I Review I Constants I Periodic Table
The complete combustion of octane, Cg H18, a
component of gasoline, proceeds as follows:
2C3H18 (1) + 2502 (g)→16CO2(g) + 18H2O(g)
Part A
Relevant volumetric equivalencies
How many moles of O2 are needed to burn 1.65 mol of C3 H18?
1 gal = 3.785 L
1 L = 1000 mL
Express the amount in moles to three significant digits.
ΑΣφ.
?
n =
mol
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 4 attempts remaining
Part B
How many grams of O2 are needed to burn 14.0 g of Cg H18?
Express the mass in grams to three significant digits.
?
m =
g
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If someone takes 2 mg of Hg2+ per day in his food, and the steady state concentration was 9 mg, Calculate t0.5 for the pollutant in the body?arrow_forwardNitromethane (CH3NO2) burns in air to produce significant amounts of heat. 4 CH3NO2 (1) + 3 O2 (g) → 4 CO2 (g) + 6 H2O (1) + 2 N2 (g) , AH°rxn = -2836 kJ If 1,869 kJ of heat are produced from the burning of nitromethane, how many grams of nitromethane were burned? Enter your numerical answer in units of grams.arrow_forwardAccording to the nutritional information on a package of jasmine rice, each serving (1/4 cup or 45.0 g) contains 160 Calories (kcal) of energy. If 3.03 g of jasmine rice is burned in a bomb calorimeter initially at 24.1°C and having a heat capacity of 8.93 kJ /°C, what will be the final temperature (in °C) of the calorimeter when the reaction is complete? Give 3 sig figs in your answer.arrow_forward
- A 43.9 g spoon made of pure silver (c = 0.235 J/g C) at 20.1 celcius is used to stir an 8 oz. (240 g) cup of coffee at 9.12 celcius. what is the final temperature of the coffee? Assume that the coffee is an insulated container and the spoon absorbs all of the heat from the coffee, and that the coffee has the same specific heat as water.arrow_forwardEry 1 3 M (M) 2req The production capacity for acrylonitrile (C3H3N) in the United States is over 2 billion pounds per year. Acrylonitrile, the building block for polyacrylonitrile fibers and a variety of plastics, is produced from gaseous propylene, ammonia, and oxygen: 2C3H6(g) + 2NH3(g) + 30₂(g) → 2C3H3N(g) + 6H₂O(g) Assuming 100% yield, determine the mass of acrylonitrile which can be produced from the mixture below: Mass 7.28x102 g 5.00 x 102 g 1.00 x 103 g In progress Reactant oxygen propylene ammonia oxygen What mass of water is formed from your mixture? mass of water formed = g Calculate the mass (in grams) of each reactant after the reaction is complete: Submit g = mass of acrylonitrile produced Reactant Mass remaining propylene ammonia Show Hints ס ס ס g g Cengage Learning Cengage Technical Support Previous Next Save and Exitarrow_forwardSome commercial drain cleaners use a mixture of sodium hydroxide and aluminum powder. When the solid mixture is poured into the drain and dissolves, a reaction ensues that produces hydrogen gas: 2N2OH(aq) + 2Al(s) + 6H2O() – 2NAAI(OH)4(aq) + 3H2(g) Determine the temperature (in °C) of hydrogen gas produced when 31.58 g of aluminum reacts with excess sodium hydroxide and water if the pressure is 103.10 kPa and the volume is 36.23 L. Provide your answer with TWO decimals. Your Answer: Answer unitsarrow_forward
- What is the kinetic energy (in Joules) of a chlorine molecule moving at 167 m/sec?arrow_forwardCalculate the energy of combustion per gram of Olive Oil (kJ/g) if 29.9 mL of water is placed in the test tube and the temperature changes from 22.9 °C to 44.6 °C. The initial mass of the spirit burner is 204.60 g and the final mass is 204.51g. State your answer with correct significant digits. Notes: Include a negative sign if the reaction is exothermic Do not include units in your answer.arrow_forwardGive the correct answer for the attached questionarrow_forward
- DATA : Mass of Magnesium (g) 0.0525 g Volume of hydrogen gas (mL) 50.0 mL Temperature of hydrogen gas (°C) 25.0°C Atmospheric Pressure (mmHg) 762 mm Hg Vapor pressure of water (mmHg) Partial pressure of hydrogen (mmHg) DATA ANALYSISarrow_forwardA sample of gas is kept at 5 atmospheres, 25∘C, and takes up 3 L. If the gas is cooled, the volume changes to 1.5 L and the pressure changes to 4 atmospheres, what is the final temperature in degrees Celsius? Show all your work.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY