
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
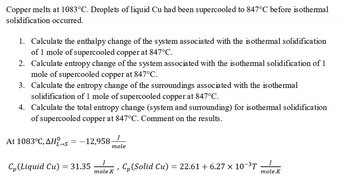
Transcribed Image Text:Copper melts at 1083°C. Droplets of liquid Cu had been supercooled to 847°C before isothermal
solidification occurred.
1. Calculate the enthalpy change of the system associated with the isothermal solidification
of 1 mole of supercooled copper at 847°C.
2. Calculate entropy change of the system associated with the isothermal solidification of 1
mole of supercooled copper at 847°C.
3. Calculate the entropy change of the surroundings associated with the isothermal
solidification of 1 mole of supercooled copper at 847°C.
4. Calculate the total entropy change (system and surrounding) for isothermal solidification
of supercooled copper at 847°C. Comment on the results.
At 1083°C, AHL-s = -12,958-
L→S
Cp (Liquid Cu)
= 31.35
mole
J
mole.K
Cp (Solid Cu) = 22.61 + 6.27 × 10-³T
)
J
mole.K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
for 1 and 2, why is it in deg C and not kelvin (K)?
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
for 1 and 2, why is it in deg C and not kelvin (K)?
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemical engineer is studying the two reactions shown in the table below. In each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 144.0°C and constant total pressure. Then, he measures the reaction enthalpy ΔH and reaction entropy ΔS of the first reaction, and the reaction enthalpy ΔH and reaction free energy ΔG of the second reaction. The results of his measurements are shown in the table. Complete the table. That is, calculate ΔG for the first reaction and ΔS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. →+C3H8g5O2g+3CO2g4H2Ol =ΔH−2220.kJ =ΔS−5322.JK =ΔGkJ Which is spontaneous? this reaction the…arrow_forwardConsider the reaction 2NH3(g) + 3N₂O(g) → 4N₂ (9) + 3H₂O(g) for which AH° = − 879.5 kJ and AS° = 288.1 J/K at 298.15 K. 1. Calculate the entropy change of the UNIVERSE when 2.344 moles of NH3(g) react under standard conditions at 298.15 K. ASuniverse = J/K 2. Is this reaction reactant or product favored under standard conditions? 3. If the reaction is product favored, is it enthalpy favored, entropy favored, or favored by both enthalpy and entropy? If the reaction is reactant favored, choose 'reactant favored'.arrow_forwardTable 2. Gibbs Free Energies of formation (kJ), ∆G°f, for Ions in 1M Solution and Ionic Solids Cations Cl--131.228 I--51.57 NO3--108.74 SO4-2-744.53 Ag+77.107 AgCl -109.789 -66.19 -33.41 -618.41 Calculated values of ∆G°rxn and the ∆Grxn of each box, Predicted results (ppt or no ppt).Observations (Rxn or No Rxn). S or support and R for Refute Cations Cl- I- NO3- SO4-2 Ag+ Ba+2arrow_forward
- a. The molar volume of a certain solid is 142.0 cm3 mol−1 at 1.00 atm and 427.15 K, its melting temperature. The molar volume of the liquid at this temperature and pressure is 152.6 cm3 mol−1. At 1.2 MPa the melting temperature changes to 429.26 K. Calculate the enthalpy (KJ/mol) and entropy of fusion (J/mol-K) of the solid. b. Calculate the melting point of ice under a pressure of 10 MPa. Assume that the density of ice under these conditions is approximately 0.915 g cm−3 and that of liquid water is 0.998 g cm−3. From the result of the calculation, what happens when you increase the pressure on water, will it freeze or will the ice melt?arrow_forwardThe vaporisation of a certain element at 25.0 oC has the following enthalpy and entropy values: Hvap = 1.00 kJ mol-1 and Svap = 156 J K-1 mol-1. What is the total entropy change (Stot) for the vaporisation of this element, in J K-1mol-1?arrow_forwardCarbon dioxide dissolves in water to form carbonic acid. Estimate the thermodynamic equilibrium constant for this reaction using the AG; values in the table. Substance AG; (kJ/mol) H,CO, (aq) -616.1 H,O(1) -237.1 CO,(g) -394.4 K = Carbonic acid then ionizes in water (K = 4.5 x 10-7). Ignoring K2, estimate K for the overall process by which CO, and H,O form H* and HCO,. K = What is the pressure of CO, in equilibrium with carbonated water at 25 °C and pH = 4.78? Роо, atmarrow_forward
- Calculate AG° and K, at 25 °C for the reaction 2 CO(g) + O2(g)→2 CO2(g) Is the reaction product-favored or reactant-favored under standard conditions? Thermodynamic Data: G'r (kJ/mol) Species CO(g) CO2(g) -137.17 -394.36 AG° = kJ/mol K, = O product-favored O reactant-favoredarrow_forwardThe complete combustion of liquid benzene is represented by the equation: C6H6(1) + 7½02(g) → 6CO2(g) + 3H20() Using the data below, calculate, for this reaction a. ΔΗ b. AS° C. AG° at 25°C. ΔΗ' kJ AS° J/K AG° kJ A +3271 -217 +3206 -3271 -217 -3206 -3271 +217 -3206 D +3271 -217 -3206 Aarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY