
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
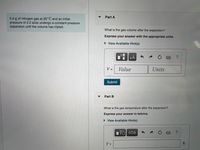
Transcribed Image Text:Part A
5.0 g of nitrogen gas at 20° C and an initial
pressure of 2.2 atm undergo a constant-pressure
expansion until the volume has tripled.
What is the gas volume after the expansion?
Express your answer with the appropriate units.
• View Available Hint(s)
µÀ
V =
Value
Units
Submit
▼
Part B
What is the gas temperature after the expansion?
Express your answer in kelvins.
• View Available Hint(s)
ΑΣφ
T =
K
Expert Solution
arrow_forward
Step 1
Given,
The amount of Nitrogen gas (m)=5.0 g
Initial temperature of the gas
Initial Pressure of the Gas (P)=2.2 atm
Final volume of the gas=Triple of Initial volume of the gas
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Part A A piston has an external pressure of 6.00 bar. How much work has been done in joules if the cylinder goes from a volume of 0.160 litres to 0.650 litres. Express your answer with the appropriate units. • View Available Hint(s) HA w = Value Units 国arrow_forwardSix thermodynamic states of the same monatomic ideal gas sample are represented in the figure. (Figure 1) Rank these states on the basis of the temperature of the gas sample in each state. Rank from largest to smallest. To rank items as equivalent, overlap them. • View Available Hint(s) Reset Help E F В A Figure 1 of 1 largest smallest F The correct ranking cannot be determined. E Submit A B Part B Complete previous part(s)arrow_forwardQ2 Help pleasearrow_forward
- Question 3 0.70 moles of an ideal gas expands adiabatiacally from 1.0 atm to 2.5 atm at a temperature of 30 Celsius. Calculate the values of QW, ΔΗ, Δ S, and Δ G. Use the editor to format your answerarrow_forwardConstants Periodic Table Assume all temperatures to be exact, and neglect significant figures for small changes in dimension. On a warm day (92 °F), an air-filled balloon occupies a volume of 0.300 m³ and has a pressure of 24.0 lb/in?. Part A If the balloon is cooled to 32 °F in a refrigerator while its pressure is reduced to 14.7 lb/in?, what is the volume of the air in the container? (Assume that the air behaves as an ideal gas.) nνα ΑΣφ V = 0.170 m3 Submit Previous Answers Request Answer X Incorrect; Try Again; 6 attempts remainingarrow_forwardA large cylindrical tank contains 0.750 m³ of nitrogen gas at 22.0°C and 6.50x103 Pa (absolute pressure). The tank has a tight-fitting piston that allows the volume to be changed. Part A What will be the pressure if the volume is decreased to 0.530 m³ and the temperature is increased to 165°C? Express your answer in pascals. —| ΑΣΦ P = Submit Request Answer < Return to Assignment Provide Feedback ? Paarrow_forward
- 5.0 g of nitrogen gas at 20°C and an initial pressure of 2.8 atm undergo a constant-pressure expansion until the volume has tripled. Part C How much heat is transferred to the gas to cause this expansion? Express your answer with the appropriate units. ► View Available Hint(s) Qexp 3000 J Submit Previous Answers ✓ Correct The gas pressure is then decreased at constant volume until the original temperature is reached. Part D What is the gas pressure after the decrease? Express your answer with the appropriate units. View Available Hint(s)arrow_forwardA cylinder measuring 8.0 cm wide and 9.5 cm high is filled with gas. The piston is pushed down with a steady force measured to be 19. N. • piston cylinder gas Calculate the pressure of the gas inside the cylinder. Write your answer in units of kilopascals. Round your answer to 2 significant digits. O kPa ?arrow_forwardWhat is the maximum mass of ethyl alcohol you could boil with 2000 J of heat, starting from 17 °C? Express your answer with the appropriate units. ► View Available Hint(s) μᾶ Value Units ?arrow_forward
- I am doing some practice problems to prepare for an exam and i cannot solve this problem, specifically part a but I would like to check this problem A piston cylinder device contains 0.8 kg of air initially at 100 kPa, 27 °C. The air is now compressed slowly in a polytropic process during which PV^1.3 =constant, until the volume reaches 1/2 its original volume. The system is then heated at constant pressure until the volume gets back to its original value. NOTE: THIS IS NOT A CYCLE. Data: Rair = 0.287 kJ/kg K; Cv=0.731 kJ/kg K; Cp=1.018 kJ/kgK. a) Determine the amounts AND directions of the Work and Heat(each in kJ) for each of the two processes. show all work used to get your values. b) Sketch the two processes on a P-v diagram, clearly showing all three state points and the two processes with their directions.arrow_forwardSubject: physicsarrow_forward• Part A A gas is in a sealed container. By what factor does the gas temperature change if the volume and pressure are both doubled? η ΑΣφ T Submit Request Answer Part B By what factor does the gas temperature change if the volume is halved and the pressure is tripled? ΑΣφ Submit Request Answer IIarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON