
Physics for Scientists and Engineers, Technology Update (No access codes included)
9th Edition
ISBN: 9781305116399
Author: Raymond A. Serway, John W. Jewett
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
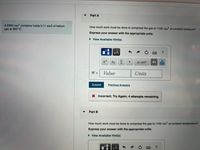
Transcribed Image Text:Part A
A 2300 cm container holds 0.11 mol of helium
How much work must be done to compress the gas to 1100 cm at constant pressure?
gas at 350° C.
Express your answer with the appropriate units.
• View Available Hint(s)
HÀ
Xb
X•10n
Value
Units
%3D
Submit
Previous Answers
X Incorrect; Try Again; 4 attempts remaining
Part B
3
How much work must be done to compress the gas to 1100 cm at constant temperature?
Express your answer with the appropriate units.
• View Available Hint(s)
HA
Expert Solution
arrow_forward
Step 1

Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A metallic container of fixed volume of 2.5103 m3 immersed in a large tank of temperature 27 contains two compartments separated by a freely movable wall. Initially, the wall is kept in place by a stopper so that there are 0.02 mol of the nitrogen gas on one side and 0.03 mol of the oxygen gas on the other side, each occupying half the volume. When the stopper is removed, the wall moves and comes to a final position. The movement of the wall is controlled so that the wall moves in infinitesimal quasi-static steps. (a) Find the final volumes of the two sides assuming the ideal gas behavior for the two gases. (b) How much work does each gas do on the other? (c) What is the change in the internal energy of each gas? (d) Find the amount of heat that enters or leaves each gas.arrow_forwardUnder what circumstances would you expect a gas to behave significantly differently than predicted by the ideal gas law?arrow_forwardSuppose a gasfilled incandescent light bulb is manufactured so that the gas inside the bulb is at atmospheric pressure when the bulb has a temperature of 20.0C. (a) Find the gauge pressure inside such a bulb when it is hot, assuming its average temperature is 60.0C (an approximation) and neglecting any change in volume due to thermal expansion or gas leaks. (b) The actual final pressure for the light bulb will be less than calculated in part (a) because the glass bulb will expand. What will the actual final pressure be, taking this into account? Is this a negligible difference?arrow_forward
- Which of the assumptions below is not made in the kinetic theory of gases? (a) The number of molecules is very large. (b) The molecules obey Newtons laws of motion. (c) The forces between molecules are long range. (d) The gas is a pure substance. (e) The average separation between molecules is large compared to their dimensions. (f) of (his account are correct statements necessary for a clear and complete explanation? (ii) Which are correct statements that are not necessary to account for the higher thermometer reading? (iii) Which are incorrect statements?arrow_forwardIn the text, it was shown that N/V=2.681025m3 for gas at STP. (a) Show that this quantity is equivalent to N/V=2.681019cm3, as stated. (b) About how many atoms are mere in one m3 (a cubic micrometer) at STP? (c) What does your answer to part (b) imply about the separation of Mama and molecules?arrow_forwardUnreasonable results. (a) Find the temperature of 0.360 kg of water, modeled as an ideal gas, at a pressure of 1.01105 Pa if it has a volume of 0.615 m3. (b) What is unreasonable about this answer? How could you get a better answer?arrow_forward
- An ideal gas goes from state (pi,vi,) to state (pf,vf,) when it is allowed to expand freely. Is it possible to represent the actual process on a pVdiagram? Explain.arrow_forwardPls explain it in terms of P and V also if possible and in detail im a little weak.arrow_forwardThe figure is a histogram showing the speeds of the molecules in a very small gas. (Figure 1) Figure N 4- 3 2 ili. 4 6 8 2 v (m/s) What is the most probable speed? Express your answer with the appropriate units. Value Submit Part B μA Submit Value Part C What is the average speed? Express your answer with the appropriate units. μÅ • Request Answer Value Units μÅ 4 Units Request Answer ? What is the rms speed? Express your answer with the appropriate units. Units ? ?arrow_forward
- • Part A A gas is in a sealed container. By what factor does the gas temperature change if the volume and pressure are both doubled? η ΑΣφ T Submit Request Answer Part B By what factor does the gas temperature change if the volume is halved and the pressure is tripled? ΑΣφ Submit Request Answer IIarrow_forwardWhat is the rms speed of argon atoms at 845 °C? Express your answer with the appropriate units. ▸ View Available Hint(s) Submit Part B HA Value Units ? What is the rms speeds of hydrogen molecules at 845 °C? Express your answer with the appropriate units. ہےarrow_forwardarrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning


College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning