
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
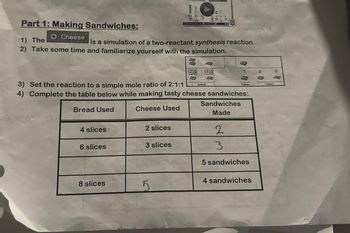
Transcribed Image Text:Part 1: Making Sandwiches:
O Cheese
4 slices
1) The
is a simulation of a two-reactant synthesis reaction.
2) Take some time and familiarize yourself with the simulation.
6 slices
8 slices
3) Set the reaction to a simple mole ratio of 2:1:1
4) Complete the table below while making tasty cheese sandwiches:
Bread Used
Cheese Used
2 slices
3 slices
28 02
5
AD
Reactants
PRE
5
2
3
Sandwiches
Made
Products
5 sandwiches
4 sandwiches
Leftovers
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the chemical equation to find the answers. H2CO3 → H2O + CO2 Identify how many oxygen atoms are in the product. Identify how many oxygen atoms are in the reactant.arrow_forwardAre chemical reactions reversible? In other words, is it possible the reverse the direction of a reaction to convert the reaction products back into reactants?arrow_forwardClassify each chemical reaction: answer must be one of the options in the drop down listarrow_forward
- Please help!arrow_forwardHydrogen peroxide decomposes as shown below: 2 H2O2 → 2 H2O + O2 molar mass H2O2 = (34.0 g/mol) A sample of hydrogen peroxide solution was analyzed using the procedure from the “Enzymatic Decomposition and Analysis of Hydrogen Peroxide” experiment. A 7.54 g sample of the solution generated 0.0277 mol of oxygen gas. Calculate the mass percent of H2O2 in the solution.arrow_forwardNonearrow_forward
- Please send me the question in 20 minutes it's very urgent plzarrow_forwardWhich equation shows a decomposition reaction? A B C D O Search CH +0 4(g) 2(g) CaO () Mg(s) PbCO 4 +02(8) + CO2(g) → → CO Beno = CO 2(8) 0 0 0 0 0 0 0 0 0 0 0 0 0 0 FEE → CaCO Mg0(s) 2 + H₂O(1) PbO (s) + CO2(g) 3(s) My UPMC o ர் -12788578- (up) for billing VAS Service Fee (0) VAS Service Fee Credits El all Que UPG Freight code- Stock charge WC 3 or 4 For will call Fema-Bran PW-Brandy! SID# 660 15arrow_forward______ In a chemical reaction the limiting reactant determines how fast the reaction will occur. Group of answer choices False Truearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY