
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
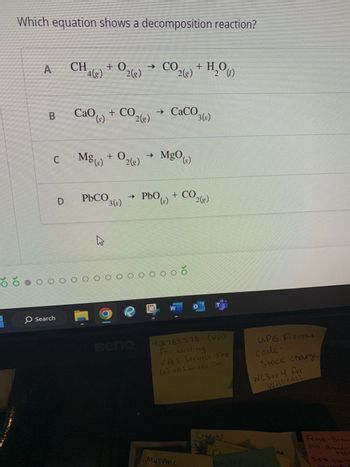
Transcribed Image Text:Which equation shows a decomposition reaction?
A
B
C
D
O Search
CH +0
4(g) 2(g)
CaO ()
Mg(s)
PbCO
4
+02(8)
+ CO2(g)
→
→ CO
Beno
=
CO 2(8)
0 0 0 0 0 0 0 0 0 0 0 0 0 0
FEE
→ CaCO
Mg0(s)
2
+ H₂O(1)
PbO (s) + CO2(g)
3(s)
My UPMC
o ர்
-12788578- (up)
for billing
VAS Service Fee
(0) VAS Service Fee
Credits
El
all Que
UPG Freight
code-
Stock charge
WC 3 or 4 For
will call
Fema-Bran
PW-Brandy!
SID# 660 15
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Balance the following equation: Fe(s) + O2(g)——-> Fe2O3(s)arrow_forwardIn the reaction, Cu + 2 AGNO3 → Cu(NO3), + 2 Ag, the substance that becomes oxidized is Cu Ag O Ag+ O Cu2+arrow_forward3.77 g of potassium is placed in 375 ml of 0.850 mol/l chromium (iii) nitrate solution as follows 3 k (s) + cr(no3)3 (aq) d 3 kno3 (aq) + cr (s) determine which reactant is the limiting reagent and calculate the mass, in grams, of cr produced.arrow_forward
- Balance the cquation C,H18() + 0>(g)→ CO,(g) + H;O(g) CO,(g) + H,0(g)arrow_forwardIn a blast furnace, coke, which is solid carbon, reacts with O2(g) to form carbon monoxide. The carbon monoxide then reacts with Fe2O3(s) to produce solid iron and carbon dioxide. Which of the following is the balanced equation for this combined process? O2 Fe2O3(s) + 3 C(s) + 3 02(g)->2 Fe(s) + 3 CO2(g) O2 Fe2O3(s) + 2 C(s) + 3 02(g)-> 4 Fe(s) + 2 CO2(g) O2 Fe2O3(s) + 6 C(s) + 3 02(g)--> 4 Fe(s) + 6 CO2(g) O2 Fe2O3(s) + C(s) + 3 O2(g) -> Fe(s) + CO2(g)arrow_forwardThe chemical equations below show reactions similar to those you have met already. One of them is balanced correctly, and three are not. Identify which one is correctly balanced. State why. - 4 Fe (s) + 3 O2 (g) = 2 Fe2O3 (s) - Mg (s) + O2 (g) = 2 MgO (s) - 2 K (s) + 2 O2 (g) = 2 K2O (s) - 2 Na (s) + H2O (l) = 2 NaOH + H2 (g)arrow_forward
- Balance the chemical equation below using the smallest possible whole number stoichiometric coefficients. ? Br₂ (1) + 1₂ (s) → IBr; (g) X On Ś 8arrow_forwardNitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. In the first step, nitrogen and hydrogen react to form ammonia: N,(0) + 3 H,(9) → 2 NH,(9) ΔΗ-92. k In the second step, ammonia and oxygen react to form nitric acid and water: NH,(9) + 20,(9) → HNO3(g) + H,O(g) AH=-330. kJ Calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. Round your answer to the nearest kJ. ?arrow_forward(c) Ba(CIO3)2 → BaCl2 + O2 Reaction type: (d) CrCl3+ AGNO3 Cr(NO3)3+ ABCI Reaction type: (e) H2O2 H2O + O2 Reaction type:arrow_forward
- Balance the following equation. If the coefficient is "1," you must enter the 1. C2H5NH2(1) + 02(g) --> CO2(g) + H20(1) + N2(g)arrow_forwardWhen heated, metal hydroxides decompose to produce a metal oxide and water. Selected the correct balanced equation for the decomposition of calcium hydroxide. CaOH (s) → CaO2 (s) + H2O (g) Ca(OH)2 (s) → CaO (s) + H2O (g) 2 CaOH (s) → 2 CaO (s) + H2O (g) 3 Ca(OH)2 (s) → 3 CaO2 (s) + H2O (g)arrow_forwardHello, I need help understanding how to properly balance this equation.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY