
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
The P-V diagram relates to a fixed quantity of O2, assumed to be an ideal gas. The temperature at point C is 140oC.
What is the heat added to the gas over the ABCA cycle?
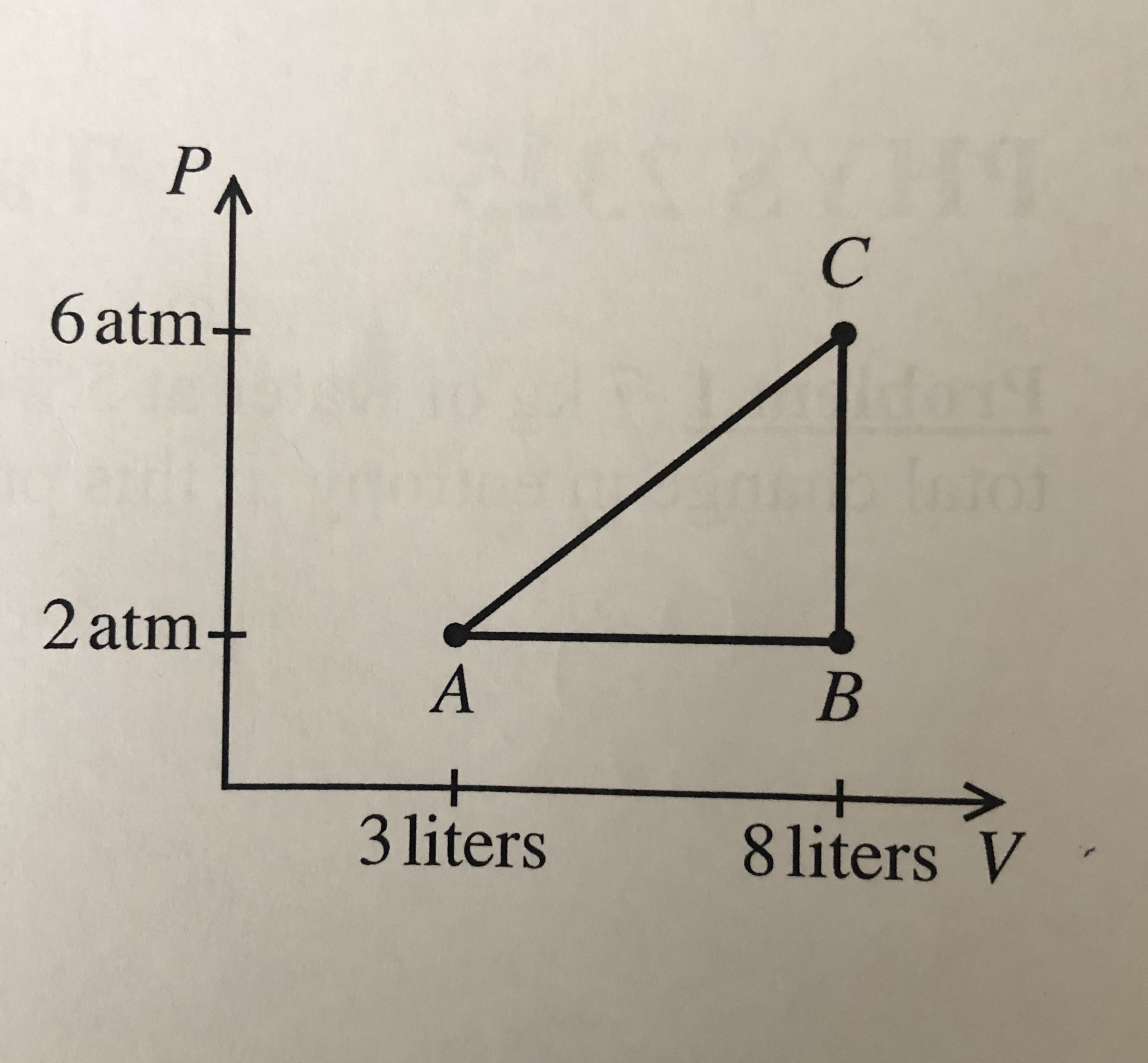
Transcribed Image Text:PA
C'
6 atm+
dont
Intol
2 atm-
A
8 liters V
3 liters
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A diatomic ideal gas at pressure p and volume V is expanding to three times its initial volume under constant pressure. in terms of p and v, calculate the heat Q flowing into the gas. 5 pV comes as wrongarrow_forwardAn ideal gas expands from state (p1, V1) to state (p2, V2) with p2=2p1 and V2=2V1. The expansion is described by p=p1[1+(V-V1)2/V21] in the pV diagram.arrow_forwardshow that the thermal eqilibirium are :arrow_forward
- One mole of an ideal monatomic gas is transferred from state a to state b along one of three paths (a → b, a → c→ b, or a →d → b). For each path find the work done by the gas and the heat absorbed. P b 2Po Po Vo 2Vo Varrow_forwardThe figure shows two closed cycles on p-V diagrams for a gas. The three parts of cycle 1 are of the same length and shape as those of cycle 2. V (1) (2) For each cycle, should the cycle be traversed clockwise or counter clockwise if the net work W done by the gas is to be positive? V (1) (2) Cycle 1 Cycle 2 Clockwise Counterclockwisearrow_forwardAn monoatomic ideal gas at 27 is in the process of constant volume and constant pressure process as in the While this is changed from 1 liter pressure 2 atm to 2 liter pressure 1 atm. Heat Capacity and Specific heat of an ideal gas. What is the final temperature of gas?arrow_forward
- A monoatomic ideal gas is taken through the cycle A → B → C → A shown in the figure. If we know that the internal energy of the monoatomic ideal gas remains constant during the process B → C, what must be the pressure pC at point C in terms of the original pressure p0? Express the work WBC done by the gas during the process B → C in terms of p0 and V0. Express the heat QBC flowing into the gas during the process B → C in terms of p0 and V0.arrow_forwardAn monoatomic ideal gas at 27 is in the process of constant volume and constant pressure process as in the While this is changed from 1 liter pressure 2 atm to 2 liter pressure 1 atm. Heat Capacity and Specific heat of an ideal gas. What is the final temperature of gas?arrow_forwardProblem 11: A diatomic ideal gas goes through the cycle a → b → c → d → a as shown in the figure. Processes ab and cd are isothermal and occur at temperatures TH = 390 K and TC = 288K, respectively. There are n = 45 moles of this gas in the system, and the initial volume is Va = 0.079 m3. Calculate the total work W done in the entire cycle, in joules.arrow_forward
- Complete the following statement: The internal energy of an ideal monatomic gas is O independent of the number of moles of the gas. O proportional to the Kelvin temperature of the gas. O a constant that is independent of pressure, volume or temperature. O dependent on both the pressure and the temperature of the gas. O proportional to the pressure and inversely proportional to the volume of the gas.arrow_forwardConsider the four-process cycle shown in the P-V diagram in the figure below. The graph shows a sequence of four processes being carried out on a sealed system of ideal gas. In this case, P is 50.0 kPa and Vis 4.00 liters. Pressure (kPa) 4P- 3P- 2P- P 2 13 14 0 V 2V 3V 4V Volume (liters) (a) Calculate the work done by the gas in the process taking the system from state 1 to state 2. 180 X J (b) Calculate the work done by the gas in the process taking the system from state 2 to state 3. 750 X J (c) Calculate the work done by the gas in the process taking the system from state 3 to state 4. 0 J (d) Calculate the work done by the gas in the process taking the system from state 4 to state 1. -750 X J (e) Calculate the net work done by the gas in one entire cycle. 180 X J (f) Calculate the net change in the internal energy of the gas for one entire cycle. 0 J (g) Calculate the net heat added to the gas for one entire cycle. 500 X Jarrow_forwardAn ideal monatomic gas is taken through the cycle in the PV diagram.(shown in photo taken) where V1 = 1.20, V2 = 2.40, P1 = 98.0 kPa and P2 = 230 kPa. If there are 0.0200 mol of this gas, What is the pressure and temperature at point C? Universal gas constant is 8.314 J/(mol·K).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON