
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:If a gas does negative work, what does the internal energy of the gas tend to do?
O increase
O decrease
O remain the same
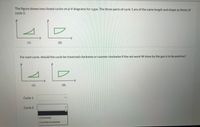
Transcribed Image Text:The figure shows two closed cycles on p-V diagrams for a gas. The three parts of cycle 1 are of the same length and shape as those of
cycle 2.
V
(1)
(2)
For each cycle, should the cycle be traversed clockwise or counter clockwise if the net work W done by the gas is to be positive?
V
(1)
(2)
Cycle 1
Cycle 2
Clockwise
Counterclockwise
<>
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Please help mearrow_forwardPlease asaparrow_forwardSuppose 6.70 mol of a diatomic gas is taken reversibly around the cycle shown in the T-S diagram of the figure, where S1 = 6.00 J/K and S2 = 8.00 J/K. The molecules do not rotate or oscillate. What is the energy transferred as heat Q for (a) path 1 to 2, (b) path 2 to 3, and (c) the full cycle? (d) What is the work W for the isothermal process? The volume V1 in state 1 is 0.420 m3. What is the volume in (e) state 2 and (f) state 3?What is the change ΔEint for (g) path 1 to 2, (h) path 2 to 3, and (i) the full cycle? (j) What is the work W for the adiabatic process?arrow_forward
- I need the answers for d-f but please also answer a-c as well.arrow_forwardA monatomic gas is take through a cycle from A to B to C and back to A. At A the pressure is 100,000 pa, the volume is 4.0 liters, and the temperature is 300K. The gas is compressed adiabatically until the volume is 1.0 liters (at B). How much energy was given to or removed from the gas during the adiabatic process?arrow_forwardWhich one of the following statements is true? (a) The path on a PV diagram always goes from the smaller volume to the larger volume. (b) The path on a PV diagram always goes from the smaller pressure to the larger pressure. (c) The area under the path on a PV diagram is always equal to the work done on a gas. (d) The area under the path on a PV diagram is always equal in magnitude to the work done on a gas.arrow_forward
- In the figure below, the change in internal energy of a gas that is taken from A to C along the blue path is +870 J. The work done on the gas along the red path ABC is -520 J. (a) How much energy must be added to the system by heat as it goes from A through B to C? (b) If the pressure at point A is five times that of point C, what is the work done on the system in going from C to D? (c) What is the energy exchanged with the surroundings by heat as the gas goes from C to A along the green path? (d) If the change in internal energy in going from point D to point A is +555 J, how much energy must be added to the system by heat as it goes from point C to point D?arrow_forwardThe graph shown is for a dilute gas that follows the clockwise path of quasi-static steps: isobaric expansion, isochoric reduction of pressure, isobaric compression, and isochoric increase in pressure. The vertical axis is shown in multiples of the pressure pp, where p=2.5atm, and the horizontal axis is shown in multiples of V, where V=4L. Part (a) What is the work done for the segment from state A to state B? Part (b) What is the work done for the segment from state B to state C? Part (c) What is the work done for the segment from state C to state D? Part (d) What is the total work done in making a single clockwise cycle, A to B to C to D?arrow_forwarda) Consider a process involving an ideal diatomic gas with n = 3mol, following p = aV, where a = 1 x 105 Pa/m³ is a constant. The gas ex- pands from volume V; = 1 m³ to V; = 4m3. P2 Find the (i) work done on the gas. (ii) heat entering the gas. 1 (iii) change in the internal energy of the gas. b) Now consider the cycle depicted in the figure, involving the same amount of gas as in the previous part. A → B is the process described in the previous subtask, B → C an isochor and C → A an isobar. Additionally, V2/V1 = n = 4 and Vi = 1 m³. Find the Pi 3 i) work done by the gas during one loop of the cycle. V1 V2 V ii) thermal efficiency of the cycle. iii) maximum theoretical efficiency of a Car- not cycle having the same temperature extrema as in this cycle. iv) coefficient of performance of the cycle, if it were used as a refrigerator .arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON