
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
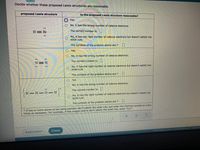
Transcribed Image Text:Decide whether these proposed Lewis structures are reasonable.
proposed Lewis structure
Is the proposed Lewis structure reasonable?
O Yes.
O No, it has the wrong number of valence electrons.
H Br
The correct number is:|
No, it has the right number of valence electrons but doesn't satisfy the
octet rule.
The symbols of the problem atoms are:*
Yes.
No, it has the wrong number of valence electrons.
N=N
The correct number is:
No, it has the right number of valence electrons but doesn't satisfy the
octet rule.
The symbols of the problem atoms are:*
O Yes.
No, it has the wrong number of valence electrons.
The correct number is:
H –H–0 – H
No, it has the right number of valence electrons but doesn't satisfy the
octet rule.
The symbols of the problem atoms are:*
* If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many
times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0".
Explanation
Check
2021 McGraw Hill LLC. All Rights Reserved. Terms of
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- ed Lewis structure N_N =0: Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:*arrow_forwardcide whether the Lewis structure proposed for each molecule is reasonable or not. molecule 13 proposed Lewis I Br₂ structure [1-3] BH [H-B-H]* Br-I- Br: Is this a reasonable structure? If not, why not? Yes, it's a reasonable structure. No, the total number of valence electrons is wrong. The correct number is: O No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: Yes, it's a reasonable structure. No, the total number of valence electrons is wrong. The correct number is: No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: Yes, it's a reasonable structure. O No, the total number of valence electrons is wrong. The correct number is: O No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: 0 * If two or more atoms have the wrong number of valence electrons around them, just enter the chemical symbol for the atom as many times as necessary. For…arrow_forwardproposed Lewis structure Is the proposed Lewis structure reasonable? O Yes. O No, it has the wrong number of valence electrons.. H-C N :F OF: O The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* O Yes. ☐ O No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ O Yes. O No, it has the wrong number of valence electrons. H-CEC-H The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐arrow_forward
- Boo Draw the Lewis dot structures and the structural formulas for the following molecules. Keep in mind the "octet" rule and its exceptions (Show lone pair electrons on the central atom when you draw the structural formula). 1. PF3 SF. BeCh HF For R Ch Tex CS2 PCI, SO, CIF; 12 iç H,0 TeCl4 BCh wwarrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. H –0 The correct number is:|| No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. ä=ö| The correct number is: || : Cl =0: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: N No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "O,0". :Z : :Z :arrow_forwardA skeletal structure for SO₂Cl₂ is shown below. Starting from this structure, complete the Lewis structure that follows the octet rule on all atoms. :O: CL II S: :CI: Click to edit moleculearrow_forward
- Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure -C=C:] H-C : 0: 0=0=0 [HO] : O OO Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* 0 Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* П Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "O,0".arrow_forwardI don't get it with this question. Can you help me, pleasearrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure : H :Z: :Z: N H I H-N-H Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* 0 Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* 0 * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0".arrow_forward
- What are covalent bonds? choose from the choices below: Bonds between atoms consisting of a shared electron pair. Bonds between a cation and an anion. Bonds between atoms of the same electronegativity. Bonds between identical atoms.arrow_forwardChoose the answer that correctly fills the blanks in the following sentence: Based on the octet rule, the element aluminum will likely form a ______ charge because it will ______. Group of answer choices 3+; lose 3 electrons Aluminum does not form an ion. 3-; gain 3 electrons 5-; gain 5 electrons 3+; gain 3 electronsarrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? ○ Yes. : 0: H- =0 =0 : C : 0: No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* O Yes. O No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* O Yes. O No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "O,O". X Garrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY