
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
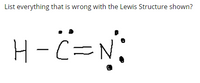
Transcribed Image Text:**Analyzing the Incorrect Lewis Structure of H-C≡N**
The image presents a Lewis structure of a molecule where a hydrogen atom (H) is bonded to a carbon atom (C), which is triple bonded to a nitrogen atom (N). The structure aims to represent the molecule hydrogen cyanide (HCN), but there are several errors in the depiction. Let's examine the issues in detail:
1. **Incomplete Valence Shell for Hydrogen:**
- Hydrogen should have only 2 electrons in its valence shell, forming a single bond. The image correctly shows this part, as hydrogen bonds with carbon, sharing two electrons.
2. **Incorrect Number of Electrons Around Carbon:**
- Carbon forms a total of four covalent bonds to complete its octet. In the correct structure, carbon should form a single bond with hydrogen (H) and a triple bond with nitrogen (N), fulfilling the octet rule. The depicted structure shows the trivalent bonding configuration correctly, involving eight electrons (two from the hydrogen bond and six from the triple bond with nitrogen).
3. **Incorrect Number of Electrons Around Nitrogen:**
- Nitrogen, in a Lewis structure, should typically have three bonds and one lone pair to complete its octet. In the correct structure, nitrogen forms a triple bond with carbon and hosts one lone pair, summing to eight electrons. The depicted structure incorrectly shows two lone pairs (four electrons) around nitrogen along with the triple bond, resulting in ten electrons around nitrogen, violating the octet rule.
To correct the given structure:
- Remove one of the lone pairs on the nitrogen atom.
**Correct Lewis Structure:**
```
H - C ≡ N:
```
- Hydrogen (H) forms a single bond with carbon (C), sharing two electrons.
- Carbon (C) forms a triple bond with nitrogen (N), sharing six electrons in the bond.
- Nitrogen (N) has one lone pair of electrons, completing its octet.
In summary, the primary errors in the provided Lewis structure are related to the number of lone pairs on the nitrogen atom, leading to an incorrect total of electrons around nitrogen. By ensuring nitrogen has only one lone pair, the structure adheres to the octet rule and accurately represents the molecule hydrogen cyanide (HCN).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1-BeCl2 2-CH4 3-SF6arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure |N—N=N] : 0: :0-C H .. I :Ö: H 1 O-H + Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are: Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are: * Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are: * * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0".arrow_forwardArrange the highlighted bonds in the table below in decreasing order of polarity. That is, pick 1 for the most polar bond, pick 2 for the next most polar bond, and so on. H H H- HIC H С CIH H :O: H Н H HLOIL C bond H Н Ö——H : C :F: H H HH с HIC C HIQIH -I H Н -CIH с H Н HH H C HIO Н -H polarity (Choose one) (Choose one) (Choose one) ▼arrow_forward
- Р ed Lewis structure H-CC-H [H_H_O_H]* Η N_0_a: Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:*arrow_forwardWhat is wrong with this Lewis dot structure for the H₂CO molecule? :Ö: 0- I H-C-Harrow_forwardDraw the resonance structure showing by the arrows for the following molecules: Molecules 1 & 2 are in the image ( IMG_9740.jpg ) Molecule 3 is in the image ( IMG_9741.jpg ) Thank you!arrow_forward
- Please draw the Lewis structure for the compounds below. State whether the compound is ionic, polar covalent, or non- polar covalent. SO3 PH3 Саоarrow_forwardWhich of the following is most likely a polar covalent bond? O Na-Cl O C-N OH-H OK-F O Ca-Oarrow_forwardWhat is the correct lewis structure of formaldehyde, H₂CO? 1 H H H H H H :O: C :Ö: C H H H Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY