
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN: 9781305960060
Author: Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
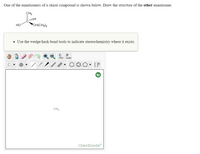
Transcribed Image Text:The image displays instructions and a chemical diagram for an educational exercise in stereochemistry:
**Text Instructions:**
"One of the enantiomers of a chiral compound is shown below. Draw the structure of the other enantiomer."
**Chemical Diagram:**
The diagram shows a chiral carbon compound with the following groups attached:
- A hydroxyl group (OH) shown with a plane bond.
- A hydrogen atom (H) shown with a wedge bond indicating it is above the plane.
- A methyl group (CH₃) shown with a dashed bond indicating it is below the plane.
- An isobutyl group (CH(CH₃)₂) shown with a plane bond.
**Additional Instruction Box:**
"- Use the wedge/hash bond tools to indicate stereochemistry where it exists."
**ChemDoodle Work Space:**
A blank canvas with a toolbar for drawing chemical structures. The toolbar includes tools for drawing bonds, atoms, and rings, indicating stereochemistry, and modifying structures. The canvas has a help icon and displays "CH₄" faintly in the center as a placeholder or example.
**Task:**
The user is tasked with using the provided tools to draw the enantiomer of the given chiral molecule, ensuring to represent the stereochemistry correctly using wedge/hash bonds.
![**One of the Cyclic Forms of D-Ribose**
The image shows the structure of one of the cyclic forms of D-ribose. This structure is a five-membered ring with four carbon atoms and one oxygen atom. The depicted form is a furanose ring. The ring includes hydroxyl (OH) groups attached to the carbon atoms.
**Structure Explanation:**
- The ring includes an ether linkage (an oxygen atom single-bonded to two carbon atoms).
- The terminal CH₂OH group extends from the anomeric carbon.
**Questions:**
(a) **How many anomeric carbon atoms are present in this structure?**
[ ]
(b) **How many hemiacetal carbon atoms are present in this structure?**
[ ]](https://content.bartleby.com/qna-images/question/75b4690e-59bd-4cfe-8e15-b27fa5bdf9fd/ec00ef70-83cf-4bee-892a-874bb02a2c67/p0jdal_thumbnail.png)
Transcribed Image Text:**One of the Cyclic Forms of D-Ribose**
The image shows the structure of one of the cyclic forms of D-ribose. This structure is a five-membered ring with four carbon atoms and one oxygen atom. The depicted form is a furanose ring. The ring includes hydroxyl (OH) groups attached to the carbon atoms.
**Structure Explanation:**
- The ring includes an ether linkage (an oxygen atom single-bonded to two carbon atoms).
- The terminal CH₂OH group extends from the anomeric carbon.
**Questions:**
(a) **How many anomeric carbon atoms are present in this structure?**
[ ]
(b) **How many hemiacetal carbon atoms are present in this structure?**
[ ]
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Similar questions
- Fats belong to the class of organic compounds represented by the general formula, RCOOR', where R and R' represent hydrocarbon groups; therefore, fats are: a. ethers. b. soaps. c. esters. d. lipases.arrow_forwardDraw the Lewis diagrams for guanine and cytosine.arrow_forwarda) Aldehydes are easily converted (oxidised) to form carboxylic acids.Ketones, on the other hand, do not oxidise to form carboxylic acids.Suggest a reason why this is so.arrow_forward
- Please don't provide handwritten solution ....arrow_forwardTo Which category does the molecule below belong? A) Protein B) alcohol C) aromatic hydrocarbons D) carbohydrate PLEASE HELP ME UNDERSTAND PLEASE PLEASE There is an image attached!arrow_forwardWhy do fatty acid esters (the biodiesel) and glycerin separate into different layers?arrow_forward
- lipid question. a. This molecule [CH2=C(CI)CH=CH2] is, in many ways, similar to isoprene. Draw this molecule's (not isoprene's) trimer, showing all elements present. CH2 groups can stay that way. No sticks, not parenthese b. You received an example of a simple polymer in the Notes. CCl,=CCl, can also form a simple polymer. Draw a tetramer derived from CC,=CC}2. I want this answer in Lewis structure format. c. Draw a tetramer of isoprene. No sticks/parentheses allowed. d. You can make polymers from all three of the above monomers [CH2=C(CI)CH=CH2, CCl2=CC12, and isoprene]. Which polymer derived from the three guys above would you expect to be most biodegradable? Explain your answer. i. ii. Which would you expect to be least biodegradable? Explain your answer.arrow_forwardA peptide bond links two amino acids together. It is an amide bond because the atoms joined by this bond are _____, and RCONR'R" is the general form of an amide.arrow_forwardWrite True or False: 1. Carbohydrates are joined together by peptide bonds. 2. A disaccharide is an example of a carbohydrate. 3. Monosaccharides are made of polysaccharides. 4. Monosaccharides have to be broken down many times during digestion. 5. Polymers are made of monomers. 6. Amylose is the most abundant biopolymer and is the basic component of cell wall of plants. 7. Every time our sugar level drops, glycogen is broken down to provide glucose in a process called syneresis. 8. If plants store glucose in the form of starch, animals store glucose in the form of glycogen. 9. Maltose is the most abundant biopolymer and is the basic component of cell wall of plants. 10. A long chain of monosaccharides linked by glycosidic bonds is known as a monosaccharide. 11. People who suffer from lactose intolerance do not produce lactase necessary to break down glucose and galactose. 12. Polysaccharides consist of a small number (around 3 to 10) of monosaccharides linked together. 13. Raffinose…arrow_forward
- Classify the functional groups in the following steroid (choose all that apply).CHOOSE ALL THAT APPLY a. alkyne b. secondary alcohol c. aldehyde d. aromatic e. alkene f. ester g. ketone h. phenol i. tertiary alcohol j. primary alcohol k. carboxylic acidarrow_forwardConsider the compound ethoxypentane and the types of organic reactions we have studied. a) Draw the chemical reaction to synthesize propanamide. **Show the structures of the reactants and products. Use Lewis structures or Condensed structures. Do not use line structures or molecular formula. b) Name the reactants. c) State the type of organic reactionarrow_forwardFor some questions you will need to use the special periodic table attached in the attached images below. Treat Je, Qu, Ap, and Bg as NONMETALS! A) Draw the condensed structural formula for the product that would be obtained when the following triacylglycerol undergoes complete hydrogenation. (Look at the attached images, also please show your work so I can understand going forward) B) Draw the condensed structural formula for 4-boguso-3-heptanethiolarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning