
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN: 9781305960060
Author: Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please don't provide handwritten solution...
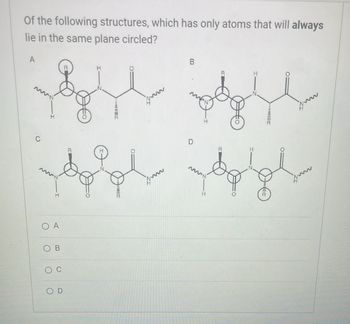
Transcribed Image Text:Of the following structures, which has only atoms that will always
lie in the same plane circled?
A
B
O
O
C
A
B
0
OD
D
R
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Draw the delocalized molecular orbitals for the following molecule. Are both bonds of the triple bond involved in the delocalized orbitals?arrow_forwardConsider the following flat drawing of methane (CH4) . a. What is HCH bond angle implied by this drawing if you assume it is flat? b. Are the electron domains of this flat CH4 spread out as much as possible? c. Use model materials to make a model of CH4 (methane). If you assembled it correctly, thefour bonds (bonding electron domains) of your model will be 109.5° apart. d. In which representation, the drawing above or the model in your hand (circle one) are theH’s of CH4 more spread out around the central carbon? e. Confirm that your model looks like the following drawing. The wedgebond represents a bond coming out of the page, and the dash bondrepresents a bond going into the page f. You will often see methane drawn as if it were flat (like on the previous page). Why is thismisleading, and what is left to the viewer’s imagination when looking at such a drawing?arrow_forwardDescribe the AXE labeling system for VSEPR in detail. What does each letter stand for? For each of the electron and molecular geometries, assign the AXE notation.arrow_forward
- How do both PH3 and H2S angle bonds compare? If different explain why How do you compre their electronic geometry and molecular geometry?arrow_forwardGroup the following molecular formulas under their respective geometries. Use the Real Molecules mode to verify the correct geometry for select structures. Drag the appropriate items to their respective bins. > View Available Hint(s) Reset Help BeCl2 SF. CO2 CH4 PCl, HCN BF3 PF, CH20 NH4+ Linear Trigonal planar Tetrahedral Trigonal bipyramidal Octahedralarrow_forwardDraw the following molecule and answer the questions below. H3CCHCHC(O)CH2CCH What is the hybridization/bonding orbital of the bolded atom? What is the hybridization of the bonded atom?arrow_forward
- Select only individual atoms, and not the bonds between them. Double bonds may be conjugated, meaning that there is one single bond between them. Identify all the atoms in the structure below that must lie in the same plane.arrow_forwardsee attachedarrow_forwardBased on the 3D structure, does the bond-line drawing match the given 3D representation? And if not draw the correct bond line structure based on the 3D representation.arrow_forward
- What orbitals are used to form each of the C–C and C – H bonds in CH3CH2CH3 (propane)? How many s bonds arepresent in this molecule?arrow_forwardWhich of the following statements are TRUE for covalent pi bonds? (Choose as many as apply. Hint: There are 4 correct answers!) Pi bonds arise from head-on overlap of two orbitals. Pi bonds arise from sideways overlap of two orbitals. The electron density in a pi bond is found above and below the axis between the two bonded nuclei. The electron density in a pi bond is found in between the two bonded nuclei. A pi bond can exist independently; not every pi bond is accompanied by an associated o bond. Pi bonds always exist in conjunction with an associated o bond; they can never exist independently. The pi bonds in a molecule determine the shape of the molecule. There can be more than one pi bond between two nuclei. Every covalent bond contains at least one pi bond.arrow_forwardIf the structure of H3 is linear, please determine MO diagram of it and bond order.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning