
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
The major organic product in the reaction sequence below is:
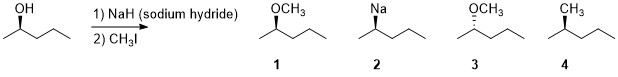
Transcribed Image Text:OCH3
Na
осн,
CHз
Он
1) NaH (sodium hydride)
2) CH3I
2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hh.182.arrow_forwardWhich of the following is a major product of the reaction shown? (CH3),CHCH;NH2 **** HN- H* (cat./H;0 (A) (B) N- (C) (D) Compound D OCompound C O Compound A Compound Barrow_forward6. Match the reagents and condition below with the appropriate reactant.You may list a reagent or condition more than once.Reagents/condition (s) : Cl2; CCl4; FeCl3; uv light; NaOH(aq); HCN(g), (i) But-1-ene : ……………………………………………………………..(ii) 1-chloroethane : ………………………………………………………...5(iii)Butane : ………………………………………………………………………(iv)Methylbenzene : ……………………………………………………………..(v) 2-chloro-2-methylpropane : ………………………………………………….(vi)Propanone : ………………………………………………………………….(vii )Ethanal : …………………………………………………………………….arrow_forward
- (4) Provide the reagents necessary to carry out the following synthesis. + enantiomer (A) (1) (i) BH3, (ii) H₂O2, NaOH; (2) NaOEt JATOT (B) (1) (i) BH3, (ii) H₂O2, NaOH; (2) TsCl, pyridine; (3) KOC(CH3)3 (C) (1) H3O+, (2) TsCl, pyridine, (3) KOC(CH3)3 (D) (1) HBr (no peroxides); (2) NaOEtarrow_forwardPlease don't provide handwritten solution...arrow_forward3) Provide the major organic product of the reaction shown below. (CH3)3 CCH2Cl, AICI3arrow_forward
- For EACH of the following reactions (a)-(d) provide a curved arrow mechanism and where appropriate briefly comment on any issues of selectivity that arise. (a) (b) (c) CHO MeO OMe 3 OH MeO NH OEt Me (i) Ph3P=CBr2 (ii) "BuLi (2 equivs) (iii) Mel + H₂SO4 (cat) OMe H₂O Me3Si * TfO OMe O MeCN CO₂Me MeO CsF, NaHCO3 Me COMe OMearrow_forward12arrow_forwardA reasonable synthesis for each of the following compoundsarrow_forward
- Give detailed Solution with explanation neededarrow_forwardThis reaction is also an important reaction of the tricarboxylic acid cycle in cells, wherein the reaction occurs in neutral solution, so the acid groups are both ionized to the carboxylate form. The reaction is catalyzed by the stereospecific enzyme fumarase that utilizes only the trans form of 2-butenedioate ion (also known as fumarate) and produces only the (S)-2-hydroxysuccinate enantiomer (also known as (S)-malate). Draw the correct stereochemical structures of these two compounds of the fumarase-catalyzed reaction. Be sure to include all hydrogen atoms and show the carboxylates as anions.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY