
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
identify the type of reactions
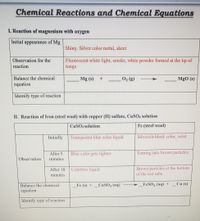
Transcribed Image Text:**Chemical Reactions and Chemical Equations**
---
**I. Reaction of Magnesium with Oxygen**
- **Initial Appearance of Mg:**
- Shiny, silver-colored metal, sheet
- **Observation for the Reaction:**
- Fluorescent white light, smoke, white powder formed at the tip of tongs
- **Balance the Chemical Equation:**
- \( \_\_\_ \, \text{Mg (s)} + \_\_\_ \, \text{O}_2 \, \text{(g)} \rightarrow \_\_\_ \, \text{MgO (s)} \)
- **Identify Type of Reaction:**
- (Blank space provided for type of reaction)
---
**II. Reaction of Iron (Steel Wool) with Copper (II) Sulfate, CuSO₄ Solution**
- **CuSO₄ Solution:**
- **Initially:** Transparent blue-colored liquid
- **After 5 Minutes:** Blue color gets lighter
- **After 10 Minutes:** Colorless liquid
- **Fe (Steel Wool):**
- **Initially:** Silverish-black color, solid
- **After 5 Minutes:** Turning into brown particles
- **After 10 Minutes:** Brown particles at the bottom of the test tube
- **Balance the Chemical Equation:**
- \( \_\_\_ \, \text{Fe (s)} + \_\_\_ \, \text{CuSO}_4 \, \text{(aq)} \rightarrow \_\_\_ \, \text{FeSO}_4 \, \text{(aq)} + \_\_\_ \, \text{Cu (s)} \)
- **Identify Type of Reaction:**
- (Blank space provided for type of reaction)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- H2O2, oxidation number of Oxygen is?arrow_forwardIn a redox reaction: electrons are created there is a transfer of protons from one species to another electrons are transferred between two species the reaction always moves in a reverse directionarrow_forwardWhen an exothermic reaction is starting: a. The reacting materials will not change their energy b.The materials in the reaction will have their highest possible energy c.Energy must be added to the reacting materials d.Energy must be removed from the reacting materials e.The reacting materials will have zero energyarrow_forward
- are the products are the things that are mixed together to start a reaction?arrow_forwardWhat type of reaction has occurred? How do you know? CH3 CH2arrow_forwardHeroin and morphine (shown below) are odorless solids. Drug-sniffing dogs can sometimes locate heroin that has been exposed to the elements because of a strong odor that is present in small quantities. What compound causes the odor and suggest what reaction is responsible for the compound's formation. H3C. но reaction? odor compound? 'N. CHз CHз H3C но herion morphinearrow_forward
- 2. If a reaction is exothermic, what will happen if the reaction is also heated? the reaction will shift left the reaction will shift right the reaction will get cooler the reaction will stoparrow_forwardEndothermic Reactions: reactants + _______= productsarrow_forwardsedibias ebnerlax To 7. Writing and Balancing Reactions. Write and Balance the following reactions with the information given. A. Solid calcium reacts with oxygen to form calcium oxide. OSHS HO AS sH+s(HO)u0- OsH B. Solid sodium hypochlorate is heated, it decomposes to form solid sodiume chloride and oxygen gas. SOSH O idal olibsen teit er ni beoubon nemels ert bns besibixo Inemele eni yhitnebl A Jnemols ert bns besiblxo Inem einu ob of tnemete noss of eledmun noilsbixo C. Hydrogen sulfide gas is passed over solid hot lead (III) hydroxide, the resultant reaction produces solid lead (III) sulfide and gaseous water. ynam 1uo mont 1egaon to viearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY