
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Transcribed Image Text:O States of Matter
Interpreting a heating curve
0/5
E
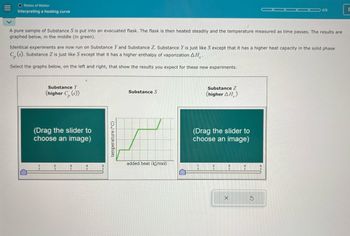
A pure sample of Substance S is put into an evacuated flask. The flask is then heated steadily and the temperature measured as time passes. The results are
graphed below, in the middle (in green).
Identical experiments are now run on Substance Y and Substance Z. Substance Y is just like S except that it has a higher heat capacity in the solid phase
C, (s). Substance Z is just like S except that it has a higher enthalpy of vaporization AH,.
Select the graphs below, on the left and right, that show the results you expect for these new experiments.
Substance Y
(higher C. (s))
(Drag the slider to
choose an image)
2
temperature (°C)
Substance S
Substance Z
(higher AH)
(Drag the slider to
choose an image)
added heat (kJ/mol)
2
X
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 1 steps with 2 images

Knowledge Booster
Similar questions
- 12 ounces of water are heated during the preparation of a cup of coffee. 1150 J of heat are added to the water, which is initially at 20 °C. What is the final temperature of the coffee?arrow_forwardA 25.0 mL sample of Benzene at 19.9 degrees celcius was cooled to its melting point, 5.5 degrees celcius and then frozen. How much energy was given off as heat in this process?arrow_forward1,922 Joules of heat are added to 42.5 grams of water, initially at 20∘C. What is the final temperature of the water?arrow_forward
- Learning Goal: To understand the concepts of heat capacity, specific heat, and molar heat capacity. Heat capacity, C, is the amount of energy required to raise the temperature of a substance by exactly one degree Celsius. The energy needed to warm an object increases as the mass of that object increases. We see this in our everyday life. For example, we know that it takes much more energy to heat a large tank of water than a small cup. Because of this dependence on mass, experimentally determined heat capacities are always reported in terms of the amount of the substance that is heated. One method is to report how much energy it takes to raise the temperature of one mole of a substance by exactly one degree Celsius. This value is the molar heat capacity, which has the symbol Cp. The molar heat capacity is given in the units J mol- °C-1.A second method is to report how much energy it takes to raise the temperature of one gram of a substance by exactly one degree Celsius. This value is…arrow_forwardbe sure your answer is correct and have the correct number of significant digitsarrow_forwardEnergy cannot be created nor destroyed, but it can be transferred between a system and its surroundings. The change in internal energy, AU, is positive if the system absorbs energy, and it is negative if the system releases energy. (Figure 1) The total change in internal energy is the sum of the heat, q, and work, w: AU=q+w. igure AU Allgyntem >0 Al system ▼ Part A Classify the following by the sign of AU for the system. Drag the appropriate items to their respective bins. If no definitive classification can be made, drag the item into the bin labeled "Not enough data." ▸ View Available Hint(s) Negative The system expands and the surroundings get hotter Submit ✓ Correct Part B Submit Previous Answers AU- Value Provide Feedback Positive μA The system contracts and the surroundings get colder. A gaseous reaction occurs at a constant pressure of 30.0 atm and releases 57.1 kJ of heat. Before the reaction, the volume of the system was 6.80 L. After the reaction, the volume of the system…arrow_forward
- Parrow_forwardIf 3.00 kJ of heat is supplied to a 0.880 mol sample of solid copper at 25.0°C, what will the copper’s final temperature be in °C? (The specific heat of solid copper is 0.385 J/g • K.)arrow_forwardA 102.8 g sample of a metal initially at 183.00C is placed in 35.0 ml of water initially at 25.0 0C. After reaching thermal equilibrium, the final temperature of the system is 58.80C. What is the identity of the metal? Assume no heat is lost to the surroundings.arrow_forward
- A piece of unknown metal with a mass of 30g is heated to 110 degrees celsius and dropped into 100g of water at 20 degrees celsius. The final temperature of the system is 25 degrees celsius. Determine the specific heat of the metal.arrow_forwardPlease don't provide handwritten solution.....arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY