
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
So you escaped the fate of wrapping hamburgers. The next step is to continue proving that
you know dimensional analysis (it’s never too late for the hamburger people to come get you!)
The great thing about dimensional analysis is that sometimes you can solve problems without
equations. Here is such a case:
a. All matter has a property called a specific heat capacity. For silver, this specific heat
capacity is 0.24 J/°C · g. How much energy (in Joules) would be required to heat 120.0 g of
silver (Ag) so that its temperature changes by 32°C? Use dimensional analysis, not an
equation!
you know dimensional analysis (it’s never too late for the hamburger people to come get you!)
The great thing about dimensional analysis is that sometimes you can solve problems without
equations. Here is such a case:
a. All matter has a property called a specific heat capacity. For silver, this specific heat
capacity is 0.24 J/°C · g. How much energy (in Joules) would be required to heat 120.0 g of
silver (Ag) so that its temperature changes by 32°C? Use dimensional analysis, not an
equation!
Dimensional Analysis Practice Problems
Page 6 of 6
b. Based on how you set up the problem above, what would be the equation? Fill in the rest of
this expression to form your own equation (that you figured out by using dimensional analysis
above.) You will use the terms “mass” “specific heat” and “temperature” and some
mathematical operation signs). This answer is an equation, not a dimensional analysis setup!
Page 6 of 6
b. Based on how you set up the problem above, what would be the equation? Fill in the rest of
this expression to form your own equation (that you figured out by using dimensional analysis
above.) You will use the terms “mass” “specific heat” and “temperature” and some
mathematical operation signs). This answer is an equation, not a dimensional analysis setup!
Expert Solution
arrow_forward
Step 1
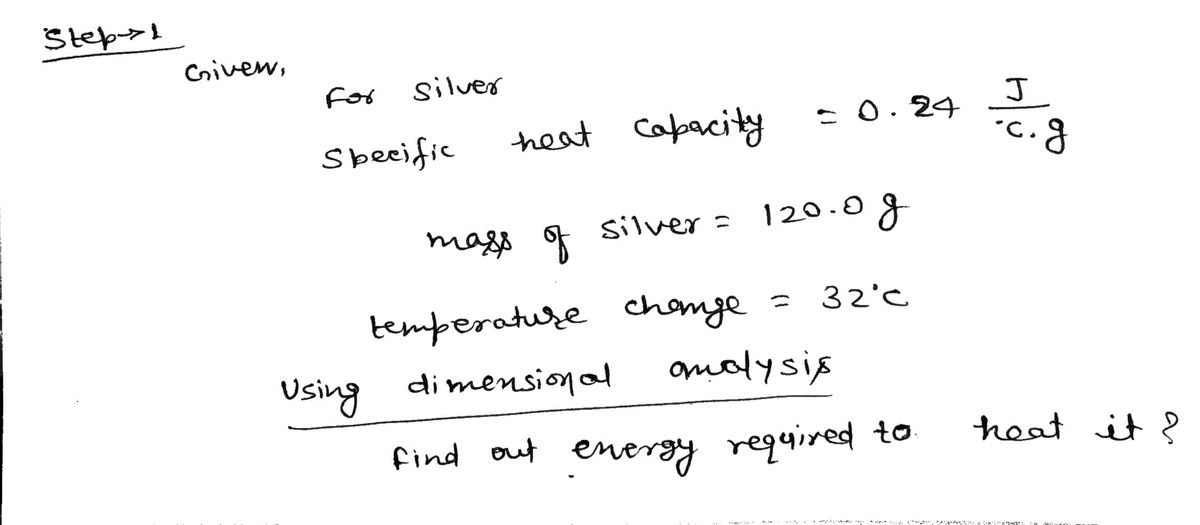
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- It takes 2.16 J of heat to raise the temperature of Object A by 1 °C, and 4.64 J to raise the temperature of Object B by 1 °C. Suppose A and B are brought into contact. A is initially hotter. A is seen to cool down by 6.9 °C. How would you calculate the rise in temperature of B? Set the math up. But don't do any of it. Just leave your answer as a math expression. Also, be sure your answer includes all the correct unit symbols. change in temperature of B = 0 Continue OO X 9 0.0 3 wevee muuiầw Hill LLC. All Rights Reserved. Terms of Use Submit Assignment Accessibility Privacy Center ? doarrow_forwardIn model 1 of the activity 'Vapor Pressure vs. Temperature and Heating Curves and Vapor Pressure, what does the slope of line segments 1 and 3 of the heating curve represent? O the enthalpy of vaporization or enthalpy of fusion different masses are used when recording the temperature change different masses are used when recording the temperature change the specific heat capacity of the state being heatedarrow_forwardthermometer. insulated container A sample of quartz, which has a specific heat capacity of 0.730 J-goC, is put into a calorimeter (see sketch at right) that contains 300.0 g of water. The quartz sample starts off at 90.0 °C and the temperature of the water starts off at 25.0 °C. When the temperature of the water stops changing it's 27.1 °C. The pressure remains constant at 1 atm. water Calculate the mass of the quartz sample. Be sure your answer is rounded to 2 significant digits. 0 x10 X ? a sample a calorimeter do Farrow_forward
- Thermochemistry Using specific heat capacity to find heat Calculate the energy required to heat 0.70 kg of silver from -3.2 °C to 4.4 °C. Assume the specific heat capacity of silver under these conditions is -1 0.235 J.g¹·K ¹. Round your answer to 2 significant digits. x10 μ ☑ 0/3arrow_forwardEnergy requirements to convert an ice cube at -10.0 °C to liquid water at +10.0 °C Energy required to raise temperature of solid: q1mCs, solid T Energy required to convert from solid to liquid: qz = nΔΗ fusion Energy required to raise temperature of liquid: 93 = MCs, liquid T Added Thermal Energy Note: you should be familiar with all of the variables and units in these equations from the last chapter of material on Thermochemistry (Tro, chapter 6). The only new variable is AHfusion, which is the enthalpy change for the melting (fusion) of a solid. Temperature (°C) 10 8 -4 6 00 Model 1 continued. -6 -8 -10 1 solid → liquid phase transition 2 10 °C temperature change of solid 3 10 °C temperature change of liquidarrow_forwardCalculate the energy required to heat 718.0 mg of water from 46.0 °C to 56.7 °C. Assume the specific heat capacity of water under these conditions is -1 -1 4.18 J∙g¯¹·K-¹ Be sure your answer has the correct number of significant digits. 0 x10 X μ 010 ? 00. 18 Ararrow_forward
- Calculate the energy required to heat 1.10 kg of water from 49.1 °C to 62.6 °C. Assume the specific heat capacity of water under these conditions is 4.18 J-g ¹K¹. Round your answer to 3 significant digits. 1 H X 10 S 5 ob Ararrow_forwardTogether, a pure gold ring and a pure titanium ring have a mass of 15.07 g. Both rings are heated to 94.90 °C and dropped into 13.0 mL of water at 16.80 °C. The water and the rings reach thermal equilibrium at a temperature of 24.20 °C. The density of water is 0.998 g/mL... The specific heat capacity of water is 4.18, the specific heat capacity of gold is 0.129 and the specific heat capacity of titanium is 0.544 Calculate the mass of each ring. mass of gold ring: mass of titanium ring: 50 60arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY