
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
It takes 572 kJ of energy to decompose 2.00 mol of liquid water. How much energy does it take to decompose 39.9 mol of water?
2H2O(l)+572kJ→2H2(g)+O2(g)
Express your answer to three significant figures and include the appropriate units.
Expert Solution
arrow_forward
Step 1
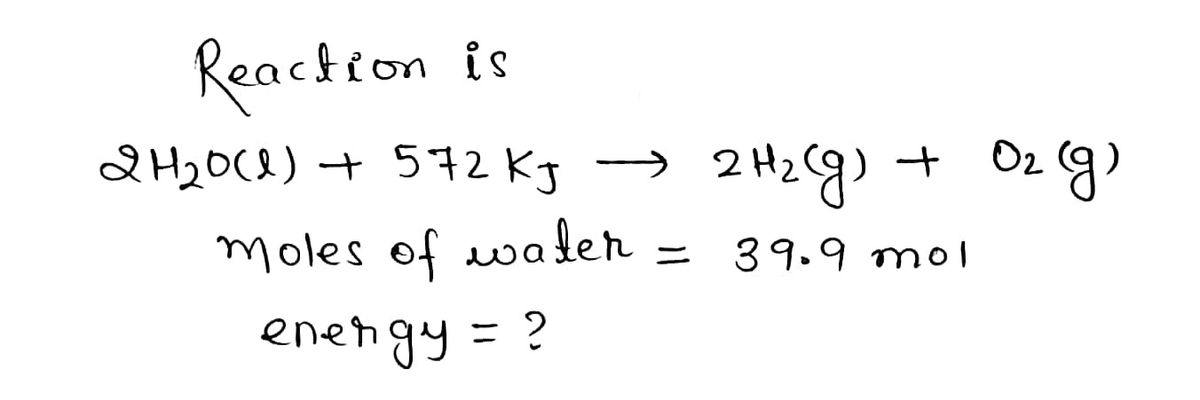
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using Hess's Law to calculate net reaction enthalpy Nitric oxide (NO) can be formed from nitrogen, hydrogen and oxygen in two steps. In the first step, nitrogen and hydrogen react to form ammonia: N₂(g) + 3H₂(g) → 2NH3(g) In the second step, ammonia and oxygen react to form nitric oxide and water: 4 NH3(g) + 50₂(g) → 4NO(g) + 6H₂O(g) ΔΗ= - 905. kJ ΔΗ= - 92. kJ Calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. Round your answer to the nearest kJ. kJ X Sarrow_forwardTo treat a burn on his hand, a person decides to place an ice cube on the burned skin. The mass of the ice cube is 12.7 g,12.7 g, and its initial temperature is −14.4 ∘C.−14.4 ∘C. The water resulting from the melted ice reaches the temperature of his skin, 29.8 ∘C.29.8 ∘C. How much heat is absorbed by the ice cube and resulting water? Assume that all of the water remains in the hand. q= Jarrow_forwardA 55.1g sample of polystyrene, which has a specific heat capacity of 1.880·J·g−1°C−1, is put into a calorimeter (see sketch at right) that contains 200.0g of water. The temperature of the water starts off at 25.0°C. When the temperature of the water stops changing it's 31.9°C. The pressure remains constant at 1atm . Calculate the initial temperature of the polystyrene sample. Be sure your answer is rounded to the correct number of significant digits.arrow_forward
- A chemist carefully measures the amount of heat needed to raise the temperature of a 1.27kg sample of C4H6O2 from 41.3°C to 60.6°C. The experiment shows that 3.87 × 10^4J of heat are needed. What can the chemist report for the molar heat capacity of C4H6O2? Round your answer to 3 significant digits. = J⋅mol−1K−1arrow_forwardChlorofluorocarbons are commonly used as Freon in refrigerators and air conditioners. Cooling the average dorm room from 35°C to 22°C requires the air conditioner to convert ~2700 g of C2Cl3F3 from a liquid at 24.7 °C to a gas at 87.0 °C. How much energy in kJ is absorbed by the chlorofluorocarbon when cooling this room? The chlorofluorocarbon C2Cl3F3 has a normal boiling point of 47.6 °C. The specific heat of the liquid is 0.91 J/g⋅K. The specific heat of the gas is 0.67 J/g⋅K. The heat of vaporization is 27.49 kJ/mol.arrow_forwardA chemist carefully measures the amount of heat needed to raise the temperature of a 155.0mg sample of C9H10O2 from 46.5°C to 66.3°C . The experiment shows that 4.80J of heat are needed. What can the chemist report for the molar heat capacity of C9H10O2 ? Be sure your answer has the correct number of significant digits. ⋅J⋅mol−1K−1arrow_forward
- Calculate the energy required to heat 0.30kg of graphite from −4.8°C to 13.1°C . Assume the specific heat capacity of graphite under these conditions is 0.710J·g−1K−1 . Be sure your answer has the correct number of significant digits.arrow_forwardThe temperature of a sample of iron increased by 22.0 °C22.0 °C when 269 J269 J of heat was applied. What is the mass of the sample? Substance Specific heat J/(g · °C) lead 0.128 silver 0.235 copper 0.385 iron 0.449 aluminum 0.903arrow_forwardConsider these reactions, where M represents a generic metal. 2 M(s) + 6HCl(aq) 2 MC13(aq) + 3H₂(g) HCl(g) HCl(aq) H₂(g) + Cl₂ (g) - MC13 (s) →→→ MC1₂ (aq) Use the given information to determine the enthalpy of the reaction 2 M(s) + 3 Cl₂(g) → 2 MC1₂ (s) 1. 2. 3. 4. AH = 2 HCl(g) AH₁ = -553.0 kJ ΔΗ, = −74.8 kJ AH3 = -1845.0 kJ AH4 = -337.0 kJ kJarrow_forward
- The combustion of 1.925 g of propanol (C₂H,OH) increases the temperature of a bomb calorimeter from 298.00 K to 302.14 K. The heat capacity of the bomb calorimeter is 15.61 kJ/K. Determine AH for the combustion of propanol to carbon dioxide gas and liquid water. kJ/mol ΔΗ = TOOLS x10arrow_forwardMeals on wheels. Meals-ready-to-eat (MREs) are military meals that can be heated on a flameless heater. The heat is produced by the following reaction: Mg(s) + 2 H2O(l) ⟶ Mg(OH)2(s) + 2H2(g) | ∆Hrxn = —353.0 kJ/mol Using the abbreviated enthalpy of formation chart below, verify that the standard enthalpy of formation given for the bolded reaction is accurate. Substance ∆Hf° (kJ/mol) Mg(g) 147.1 Mg(s) 0 Mg(OH)2(s) –924.7 H(g) 217.94 H2(g) 0 H2O(l) –285.8 H2O2(l) –187.8arrow_forwardA chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 9.50kg of water at 38.9°C. During the reaction 83.3kJ of heat flows out of the bath and into the flask. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18J·g−1·K−1. Be sure your answer has the correct number of significant digits.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY