
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
What is the major neutral organic product for the following sequence?
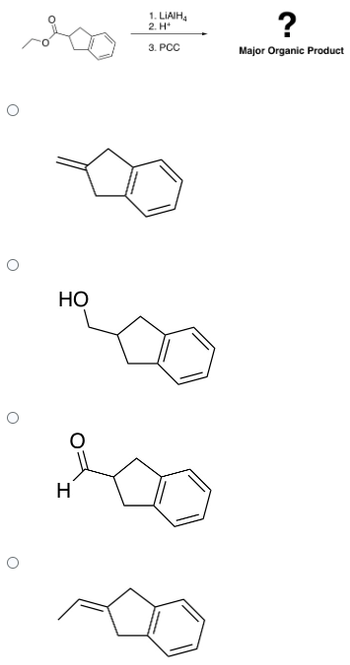
Transcribed Image Text:O
O
O
HO
H
1. LIAIH4
2. H+
3. PCC
?
Major Organic Product
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please don't provide handwriting solutionarrow_forwardWhat is the conjugated base of this equation?arrow_forwardThe reaction of an alkyl chloride with potassium iodide is generally carried out in acetone to maximize the amount of alkyl iodide that is formed. Why does the solvent increase the yield of alkyl iodide? (Hint: Potassium iodide is soluble in acetone, but potassium chloride is not.)arrow_forward
- Can you please help show how to do the syntheses for this molecule with the given reactant?arrow_forward1. Which of the following statement about safety is NOT CORRECT? A) Approved safety goggles must be worn at all times you are working in the laboratory. B) No food, drinks or smoking are allowed in the laboratory. C) Wear gloves when you are dealing with chemicals. D) Broken or cracked glassware can be used in the experiments. E) No open-toe shoes are allowed in the laboratory. 5. Which of the following is an Aldol condensation? A) Two molecules of benzaldehyde react with each other to form an alpha-hydroxy ketone B) Methanol reacts with benzoic acid to form methyl benzoate. C) Aldehydes react with methyl ketones to form alpha, beta-unsaturated ketones. D) Nitration of methyl benzoate to form methyl 3-nitrobenzoate. E) Acylation of ferrocene to form acetylferrocene. 4. Which of the following is(are) the minor product(s) in the nitration of nitrobenzene? A) Nitrobenzene B) 1,2-Dinitrobenzene C) 1,3-Dinitrobenzene D) 1,4-Dinitrobenzene E) 1,2-Dinitrobenzene and 1,4-dinitrobenzene 3.…arrow_forwardGive the major organic product for the following reactionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY