
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please give a clear image
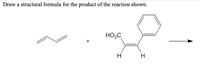
Transcribed Image Text:Draw a structural formula for the product of the reaction shown.
HO,C
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In chromatography, the compound that is least soluble in the mobile phase a) Travels the furthest away from the line of origin b) Stays closest to the line of originarrow_forwardI have tried in 4 times now and I only get one more try. I do not understand what I'm doing wrong please help me.arrow_forward4 5 6arrow_forward
- In chromatography, it is desirable to increase the resolution. a) increase the plate height (H) b) decrease the plate height (H) c) decrease the number of plates (N) toarrow_forwardOne disadvantage of thin layer chromatography is that... it cannot be used for quantitative measurements. O it consumes large volumes of solvents O it cannot analyze volatile samples it cannot be used to analyze many samples simultaneously it requires a large amount of sample for analysisarrow_forwardCompare Chromatographic and Spectroscopic methods for pharmaceutical analysis? Please answer at your own easy words. Answer should be to the point.arrow_forward
- What will happen to MP (effect and range) if one uses a melting point tube with too large a diameter?arrow_forward2- 3. Ch4 - Mastery Gmail с !!! X Canvas OWLv2 | Online teaching and l X docs.google.com/document/d/1TqDL3pNyiUclZ_7MKQ6brZDQFIAmOD1jfbX4SLstrel/edit Rangermail P University of Wisc... a se a A 100% Untitled document File Edit View Insert Format Tools Extensions Help 1 Normal text H Quizlet C chegg Verdana H I H₂N. I CI 1 I Untitled document - Google D X b Success Confirmation of Ques x + CH3 H I H 11.5 + H. H GPT P Student Center 2 -NH₂ Note that cis, trans isomers are an example of stereoisomers. Indicate whether the pair of structures shown represent stereoisomers, constitutional isomers, different conformations of the same compound, or the same conformation of a compound viewed from a different perspective. H CH3 H₂N- B I U A H I CI 3 ين - 4 Moncler NH₂| C + Pearson+ Public S... d = = ▶ Geo Book E Cengage Ochem 1 E EX 0 О н Update: ✓ Editing Share Harrow_forwardHelp 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forward
- The purpose of adding sodium sulfate in the extraction is to decrease the solubility of caffeine in dichloromethane. Hence, the residue could contain water and other impurities. a First statement is true. Second statement is false. b First statement is true. Second statement is false. c Both statements are false. d Both statements are true.arrow_forwardAssistive Technologies for S X O MYCSU - Columbus State UX Link to AL https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-JgXZp57itWHhRgilODc5MqvhZbKYx2-U-038xjApUucKTA610SL O MEASUREMENT AND MATTER Predicting the formula of ionic compounds with commo.. Write the empirical formula for at least four ionic compounds that could be formed from the following ions: CH,CO,, NH", Fe*, Cro IIarrow_forwardA ALEKS- Allie Fleming - Learn X + s.com/alekscgi/x/lsl.exe/10_u-IgNslkr7j8P3jH-IQUHIQg6bJxmeSyVpHOEB1plef9xyC5Ca9QIZnmclTUEg1JWvSyM1tTaqBJUR5NSUOCIG_6ulnLcZ71D4j2Qcp = Organic Functional Groups Understanding common names of carboxylic acids and derivatives In the drawing area below, draw the skeletal ("line") structures of propionic acid and potassium propionate. You can draw the two molecules in any arrangement you like, so long as they don't touch. Explanation $ 4 % Check 5 Click and drag to start drawing a structure. NA J- ^ Q Search (0) 6 X ♫+ & 7 fa X KAA RTY U * 8 DII 110 DDI 9 X 0:0 W 3 fm © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ODOO 0/5 10 V insert P prt sc + =arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY