
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
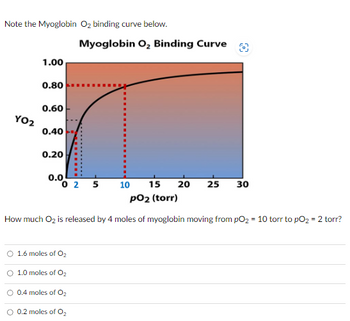
Transcribed Image Text:Note the Myoglobin O₂ binding curve below.
YO2
1.00
0.80
0.60
0.40
0.20
0.0
Myoglobin O₂ Binding Curve
0 2 5
1.6 moles of O₂
1.0 moles of O₂
0.4 moles of O₂
0.2 moles of O₂
10
15 20 25 30
po₂ (torr)
How much O₂ is released by 4 moles of myoglobin moving from pO₂ = 10 torr to pO₂ = 2 torr?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Why are enzymes important? For example, what is the importance of the enzyme carbonic anhydrase in the body?arrow_forward7-43 (Chemical Connections 7A and 7B) Why is a high fever dangerous? Why is a low body temperature dangerous?arrow_forwardBy which of the following mechanisms does a catalyst operate? a. It decreases the activation energy barrier for a reaction. b. It serves as a reactant and is consumed. c. It increases the temperature of a reaction. d. It increases the concentration of reactants.arrow_forward
- The following equation represents a reversible decomposition: CaCO3(s)CaO(s)+CO2(g) Under what conditions will decomposition in a closed container proceed to completion so that no CaCO3 remains?arrow_forwardSubstances that poison a catalyst pose a major concern for many engineering designs, including those for catalytic converters. One design option is to add materials that react with potential poisons before they reach the catalyst. Among the commonly encountered catalyst poisons are silicon and phosphorus, which typically form phosphate or silicate ions in the oxidizing environment of an engine. Group 2 elements are added to the catalyst to react with these contaminants before they reach the working portion of the catalytic converter. If estimates show that a catalytic converter will be exposed to 625 g of silicon during its lifetime, what mass of beryllium would need to be included in the design?arrow_forwardClassify each of the following statements as true or false. aSome equilibria depend on a steady supply of a reactant in order to maintain the equilibrium. bBoth forward and reverse reactions continue after equilibrium is reached. cEvery time reactant molecules collide, there is a reaction. dPotential energy during a collision is greater than potential energy before or after the collision. eThe properties of a transition state are between those of the reactants and products. fActivation energy is positive for both the forward and reverse reactions. gKinetic energy is changed to potential energy during a collision. hAn increase in temperature speeds the forward reaction but slows the reverse reaction. iA catalyst changes the steps by which a reaction is completed. jAn increase in concentration of a substance on the right-hand side of an equation speeds the reverse reaction rate. kAn increase in the concentration of a substance in an equilibrium increases the reaction rate in which the substance is a product. lReducing the volume of a gaseous equilibrium shifts the equilibrium in the direction of fewer gaseous molecules. mRaising temperature results in a shift in the forward direction of an endothermic equilibrium. nThe value of an equilibrium constant depends on temperature. oA large K indicates that an equilibrium is favored in the reverse direction.arrow_forward
- Determine rxnH 25 C for the following reaction: NO g O2 g NO2 g This reaction is a major participant in the formation of smog.arrow_forward. Hydrogen gas and chlorine gas in the presence of light react explosively to form hydrogen chloride H2(g)+Cl2(g)2HCl(g)The reaction is strongly exothermic. Would an increase in temperature for the system lend to favor or disfavor the production of hydrogen chloride?arrow_forwardAmmonia is a weak base that reacts with water according to this equation: NH3(aq)+H2O(l)NH4+(aq)+OH(aq) Will any of the following increase the percent of ammonia that is converted to the ammonium ion in water? (a) Addition of NaOH (b) Addition of HCI (c) Addition of NH4CIarrow_forward
- For each of the changes listed will the rate of the following chemical reaction increase, decrease, or remain the same? Fe(s)+2HCl(aq)FeCl2(aq)+H2(g) a. the concentration of HCl is decreased b. the iron is ground into a powder c. a catalyst is added to the reaction mixture d. the temperature of the solution is decreasedarrow_forwardWhich of the following chemical kinetic (reaction rates) factors in biological systems results in a more efficient system? Group of answer choices Decreased activation energy. Large cell size. Liquid state of cells. Solid state of cells. Lower body temperature.arrow_forwardこの 云 10 Free Energy (kcal/mol) [Review Topica] [References) 25 15 B A. Reaction progress Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, AGt, for the step B to C. kcal/mol Calculate the overall energy change, AG°, for the process A to B. kcal/mol Which step is faster, (a) C to B or (b) B to A? Submit Answer Retry Entire Group 8 more group attempts remaining MacBook Air DD 08 OL F7 F8 64 F4 F5 & * $ % 8. :]:1:/arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
