
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Provide step by step total synthesis of the following target compounds using the starting material provided.
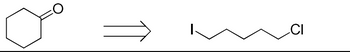
Transcribed Image Text:CI
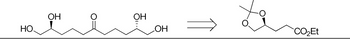
Transcribed Image Text:НО.
ОН
ОН
OH
CO₂Et
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The acetoacetic ester synthesis is a carbonyl alkylation reaction. It is used to prepare methyl ketones from primary alkyl halides, lengthening the carbon chain by three atoms. Thus, the product can be visualized as being a "substituted acetone." The reaction consists of three steps: generation of the enolate anion followed by SN2 reaction with a primary alkyl halide, ester hydrolysis under acid conditions, and decarboxylation. Br H₂C H3C 1. NaOEt 2. H₂O* 3. heat H₂C. OEt Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions H X CO2 CH3 + EtOH :OH На CH3 CO2 CH3 CH3 CH3arrow_forwardGive detailed Solution with explanation neededarrow_forwardThe acetoacetic ester synthesis is a carbonyl alkylation reaction. It is used to prepare methyl ketones from primary alkyl halides, lengthening the carbon chain by three atoms. Thus, the product can be visualized as being a "substituted acetone." The reaction consists of three steps: generation of the enolate anion followed by SN2 reaction with a primary alkyl halide, ester hydrolysis under acid conditions, and decarboxylation. м Br H3C H3C OEt 1. NaOEt 2. H₂O* 3. heat H₂C. Draw curved arrows to show the movement of electrons in this step of the mechanism. CO₂ EtOH CH3 Arrow-pushing Instructions H3C 1:0: :0: :Br: Br: H₂C CH3 CH3 H3C CH3 7barrow_forward
- Which of the following reaction conditions can be used to synthesize an ester (RCOOR)? O A carboxylic acid and an alcohol in presence of a base (base catalyzed) An acid hailide and an amine in presence of a base (base catalyzed) O An amide and an alcohol O A carboxylic acid and an alcohol in presence of an acid (acid catalyzed) - Previousarrow_forwardDraw the structures of all possible products formed from the following reaction. Specify the type of substitution or elimination pathway. Demonstrate the sterrochemical outcomes of the reaction by drawing your products in wedge dash form when necessary.arrow_forwardProvide the appropriate reagents for the examplearrow_forward
- Give the major organic product for the following reactionarrow_forward47) Provide the structure of the major organic product which results in the following reaction. Br KI Br CH3arrow_forwardPropose a multi-step synthetic route to form the polycyclic molecule on the right hand side using the following reagents on the left hand side of the arrows: i) cyclic ketone; ii) a,ẞ-unsaturated ketone; iii) either the organocuprate OR the organolithium reagent listed; and ii) any other reagents or solvents of your choosing The depicted reagents represent all necessary building blocks that are directly incorporated into the molecule on the right, however you should use additional reagents to help facilitate its construction based on Chem 110 content from Chapters 21 and 23. This is a multi-step synthesis and not all reagents will be used at the same time. When describing your solution, show the stable intermediates along your proposed synthetic route and number the individual synthetic steps. You do not have to worry about stereochemistry in this particular question. For two (2) of your proposed steps, show individual mechanistic arrows that show the flow of electrons during that…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY