
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
i dont get how to do any of this
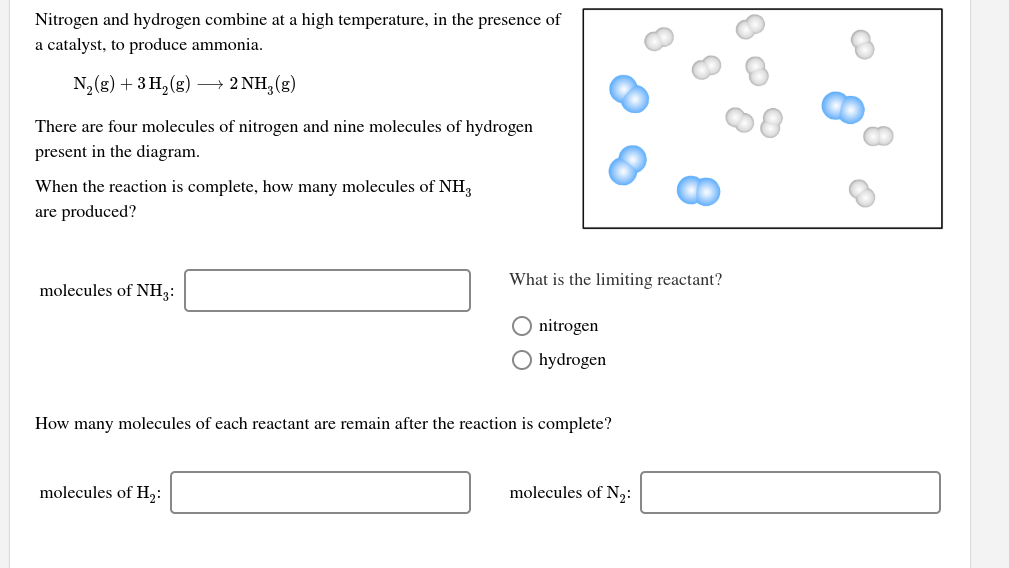
Transcribed Image Text:Nitrogen and hydrogen combine at a high temperature, in the presence of
a catalyst, to produce ammonia.
N2(g) + 3 H, (g)
→ 2 NH, (g)
There are four molecules of nitrogen and nine molecules of hydrogen
present in the diagram.
When the reaction is complete, how many molecules of NH3
are produced?
What is the limiting reactant?
molecules of NH3:
O nitrogen
O hydrogen
How many molecules of each reactant are remain after the reaction is complete?
molecules of H2:
molecules of N2:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- a) In the simulation, select the solutions indicated below from the dropdown list at the top left corner of the simulation. The beaker will fill up to the 1.00 LL mark with the solution. Arrange the acids in increasing order of acidity. Rank from lowest to highest. To rank items as equivalent, overlap them. Beer,vomit,coffee,milk,battery acid,soda pop. b)In the simulation, select the custom liquid from the dropdown list. The beaker will be filled to the 1.00 LL mark with the solution. Set the pHpH to 8.55 by entering the value in the text box provided above the pHpH scale in the panel called "pHpH." Once you adjust the pHpH, note the corresponding OH−OH− ion concentration in mol L−1mol L−1 as given in the graph on the right side of the simulation. Make sure to click the option "Concentration (mol L−1mol L−1)" under the “Water components” panel. Click on the Logarithmic scale below the graph. Find the pOHpOH of the solution.arrow_forwardA small business brings in a revenue of $60000 for the week. The payroll for the week is $20000. The owner spends $1000 on the company anniversary party. Of what is left, one third is donated to a local charity, and $500 less than half the amount that went to the charity goes to building repairs. What is the final amount of profit for the week? Question 5 options: $20000 $7000 $13000arrow_forward1) SOCI₂, Et3N OH 2) + AICI 3arrow_forward
- Which of the following statements is a feature of scaled particle theory? a) The work required to insert a solute into a solvent depends on the solute's volume only b) The work required to insert a solute into a solvent depends on the solute's accessible surface area only c) High molecular-weight compounds cross biological membranes more easily than low- molecular weight ones d) A large particle has the same surface area-to-volume ratio as a small particle e) Particles in solution pay an energetic penalty to create a cavity in the solventarrow_forwardyout References 2 v Font ₂x²A - A V Mailings Review View Help A A Aa AEE ALT Y f3 5 United States) Text Predictions: On O 14 101 === Paragraph Search (Alt+Q) 4.All are correct V Accessibility: Good to go 15 5 1.To the polarity of water and that organic compounds are nonpolar. f6 D Normal Organic compounds tend not to mix with water. What is the reason for this chemical characteristic of organic compounds with water? 2.Because water forms a hydrogen bond and hydrocarbons do not have polarity for this, unless they have an oxygen in the chain. 3.Some alkanes can mix with water if they have a carbonyl or hydroxyl group. No Spacing f7 40 Styles hp 18 Heading 1 19 0 0 f10 F17 5 F12 Find Replac Select- Editing 82°F Mosarrow_forwardHello, I would like help with problem 37 parts A and B in the image attached. Thanks!arrow_forward
- ge.com/course.html?courseld=17842654&OpenVellumHMAC=d71eb8b5068468150149000 For each of the following acid-base reactions indicate whether it will favor reactants or products. Compound pK, CH3COOH 4.8 HCOOH 3.8 15.7 PO H₂O NH3 36 H₂O+ -1.7 CH3 O H₂ -2.5 PresentationO CH_NH,+HO- =CH,NH+H,O O products. reactants Submit ▾ Part B Request Answer NH3 + H₂O + NH4+ HO™ = reactants O products Submit Request Answer P Pearson Copyright © 2023 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Parrow_forwardRsimi, Nour 2023VVA Chemistry.7b.FA1 An unknown solid substance is heated according to the temperature versus time graph below. Temperature (°C) 70 40 30 20 288 8 2 0 0 5 15 Time (min) 20 00 4 of 5 1 2 3 4 5 25 While the substance is a liquid, at what temperature do the molecules of the substance have the greatest average kinetic energy? 2 % 3 4 5 16 7 8 insertarrow_forwardYou have just started working in an Urgent Care Clinic as a nurse. Mom brings in her 3 year old little boy, Brandon. Mom states that Brandon has been having fever up to 104F and he isn't eating or drinking anything because he feels very sick to his stomach, not to mention crabby! He's also been pulling a lot at his right ear and sounds congested. At the last well visit with the Pediatrician (about 2 weeks ago), Mom states that Brandon weighed 32 pounds. On exam today, his weight is 12.5 kg. Additionally his eyes are darkened and sunken, his mouth is dry with some white crusting around the lips. His temperature is 39C, other vitals signs are normal. Though he looks ill he appears to be medically stable. The doctor rushes in, quickly exams Brandon, diagnoses him with otitis media and then barks off orders to you that you then need to convey accurately to Mom. The orders include: push fluids, ibuprofen 10mg/kg every 6 hours if temp > 38C, and begin taking a 10-day course of…arrow_forward
- Solution 1 Solution 2 Solution 3 Solution 4 Solution 5 Concentration iron(III) nitrate 0.00200 0.00200 0.00200 0.00200 0.00200 [Fe(NO3)3] (M) Concentration potassium thiocyanate 0.00200 0.00200 0.00200 0.00200 0.00200 [KSCN] (M) Volume Fe(NO3)3 (mL) 5.00 5.00 5.00 5.00 5.00 Volume KSCN (mL) 5.00 4.00 3.00 2.00 1.00 Volume DI water (mL) 0.00 1.00 2.00 3.00 4.00 Initial concentration [Fe3+] (M) 0.00100 0.00100 0.00100 0.00100 0.00100 Initial concentration [SCN] (M) 0.00100 0.000800 0.000600 0.000400 0.000200 Absorbance 0.269 0.192 0.154 0.104 0.052 Equilibrium [FeSCN2+] (M) 0.000198 0.000141 0.000113 0.0000765 0.000038 Equilibrium constant Kc Average Kcarrow_forwardWhat should I put in the boxarrow_forwardChemistry HW need help, how to do these caculation and answer these questions?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY