
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
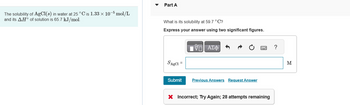
Transcribed Image Text:The solubility of AgCl(s) in water at 25 °C is 1.33 × 10-5 mol/L
and its AH° of solution is 65.7 kJ/mol.
Part A
What is its solubility at 59.7 °C?
Express your answer using two significant figures.
SABC1
Submit
17 ΑΣΦ
Previous Answers Request Answer
22
X Incorrect; Try Again; 28 attempts remaining
?
M
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- The following graph shows the solubility of KBr in water in units of grams of KBr per 100 g of H,O. If 80 g of KBr is added to 100 g of water at 10°C and the mixture is heated slowly, at what temperature will the last KBr dissolve? 100 80 60 40 20 20 40 60 80 100 Temperature (°C) Solubility (g KBr per 100 g water)arrow_forwardCalculate the solubility of Zn(OH)2 in water at 25 °C. You'll find Kp data in the ALEKS Data tab. sp Round your answer to 2 significant digits. 60 L Explanation 80°F Partly sunny Check x10 X Q Search Ⓒ2023 McGarrow_forwardN|OU|GA|OC| G b 01edu.co Maps In a_ A 2 F2 W # 3 80 F3 E > C □ $ 4 > F4 R OU Gd 2|GA|GV J|bs|G1|CS|UO A Question 29 of 31 reaction, the components of water (H-OH) are added across a carbon-carbon double bond (C=C). A) hydration B) hydrogenation C) bromination D) combustion E) elimination b 99 Aav % от бо 5 O F5 T B < 6 MacBook Air F6 Y D 7 0 F7 U * 8 DII F8 1 9 8 F9 0 0 F10 EE U Done P B Gr ☆ E 7 J F11 {arrow_forward
- Pls help ASAP.arrow_forwardAt 0 oC, the solubility of sodium acetate in water is 36.1 g per 100 g of water and at 40 oC, the solubility is 66.4 g per 100 g of water. A 100 g sample of sodium acetate is dissolved in 250 g of water at 40 °C. The solution is cooled to 0 °C and a small amount of solid is observed. This solution is ________. - hydrated -saturated -supersaturated - unsaturatedarrow_forwardConsider the Solubility Curve for this Problem: Analysis for KClO3 & NH4Cl MW (KClO3) = 122.55 g/mol , MW (NH4Cl) = 53.49 g/mol i) What is the temperature at which KClO3 & NH4Cl both have the same solubility? Round to nearest degree. +/- 1° T = °C ii) What is the solubility (g per 100g H2O) at this temperature. Round to nearest gram. +/- 1 g s (KClO3) = g/100g H2O s (NH4Cl) = g/100g H2O iii) What is the molar solubility for KClO3 at this temperature? Assume the density of the solution is 1.21 g/cc. Round off to 2 sig. figs. |s| KClO3 = M iv) Calculate the Ksp for each of these chemicals at this temperature. Ksp = v) What is the unit of Ksp for KClO3? Unit for Ksp =arrow_forward
- A sample of water is found to contain 0.012 ppm of Pb2* ions. Calculate the mass of lead ions per liter of this solution (assume the density of the water solution is 1.0 g/mL). O 5.9x10 g/L Pb2 O 1.2*10 g/L Pb O 1.2*10 11 g/L Pb2 O 1.2%10 5 g/L Pb2+ Activate Windows Go to Settings to activate Windo inspiron F10 F11 Backspac P F K Ente B Shift Alt Alt Ctrlarrow_forwardA sample of 7.63 g of Mg(OH)2 is added to 24.0 mL of 0.175 M HNO3- Part A Enter the chemical equation for the reaction that occurs. Express your answer as a balanced chemical equation. Identify all of the phases in your answer. ΑΣΦ 2¢ ? A chemical reaction does not occur for this question. Submit Request Answer Part B Complete previous part(s) Part C Complete previous part(s) Part D Complete previous part(s) Part E Complete previous part(s) Provide Feedback MacBook Pro Review Carrow_forward178. Subject :- Chemistryarrow_forward
- A solution containing 78 g of NaNO3 in 70. g H2O at 50 ° C is cooled to 20 °C. Use the solubility data from the table below. Part A Solubility (g/100. g H2O) Substance 20 °C 50 °C How many grams of NaNO3 remain in solution at 20 °C? KCI 34 43 Express your answer in grams to two significant figures. NaNO3 88 110 ? C12 H22O11 (sugar) 204 260arrow_forwardPart A How many moles of solid sodium fluoride should be added to 5.0 L of a saturated solution of barium fluoride, BaF2, at 25 °C to raise the fluoride concentration to 0.029 mol/L ? Express your answer to two significant figures and include the appropriate units. n = 0.11 ? moles Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remainingarrow_forward4- 30- [14] How many liters of the antifreeze ethylene glycol (HOCH₂CH₂OH, 54.09 g/mol) would you add to a car-radiator containing 6.50 L of water to keep it from freezing if the coldest winter temperature in your area is -20°C? Calculate the boiling point of this water-ethylene glycol mixture. (The density of ethylene glycol is 1.11 g/mL, the molal freezing and boiling point constants are respectively kf = 1.86°/m and kb= 0.52 °/m). & Page 5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY