
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:File Edit View History Bookmarks Profiles
A ALEKS-LX
Auerbach x
Mall-La X
Untitled dx A ALEKS-L X
www-awu.aleks.com/alekscgi/x/lsl.exe/10_u-IgNslkr7j8P3jH-lvTqeviKFP6W0cqJcWJdIACROQwyw24GWHIn6wmDXTUN80MO03t0lVprAPxlkn..
ity and... 18.3 Gibbs Free E... 5.3 Enthalpies of... 18.5 Gibbs Free E... Reading Schedule 19.6 Reduction Po...Ya SOLUTION: The le...
O KINETICS AND EQUILIBRIUM
Calculating an equilibrium constant from a heterogeneous...
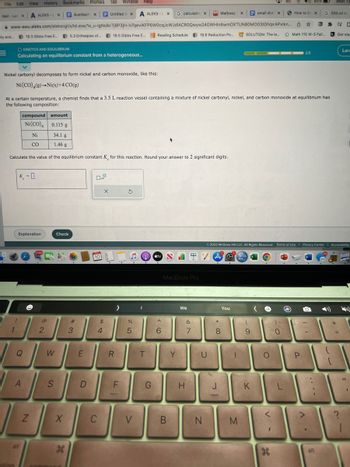
Nickel carbonyl decomposes to form nickel and carbon monoxide, like this:
Ni (CO) (9) Ni(s)+4 CO(g)
!
1
stion
compound amount
Ni (CO)4 0.115 g
K = 0
Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits.
Explanation
Q
A
all
Ni
CO
At a certain temperature, a chemist finds that a 3.5 L reaction vessel containing a mixture of nickel carbonyl, nickel, and carbon monoxide at equilibrium has
the following composition:
C
N
17.954
CO
2
B
34.1 g
1.46 g
W
comm
S
Check
X
X
#3
E
D
0.9
10
Tab Window Help
4
C
X
$
R
>
F
$
%
S
5
V
I
T
G
tv N
^
G calculating x
: 6
ST
MacBook Pro
B
Y
We
H
M Mathway X
&
7
U
N
You
8
J
email draf x
M
9
K
O
<
Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
ge
) -
0
How to En X
L
L
83%
☆ h.
Math 115 W-S Fall....
P
2/5
A.
W
G 500 ml to
alt
Mon
{
h. Get sta
+ 11
[
Lara
:-
Expert Solution
arrow_forward
Step 1
Given : reaction
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Titanium(IV) dchloride decomposes to form titanium and chlorine, like this: TiCl4(1)→Ti(s)+2 Cl,(9) At a certain temperature, a chemist finds that a 4.9 L reaction vessel containing a mixture of titanium(IV) chloride, titanium, and chlorine at equilibrium has the following composition: compound amount TiCl, 3.63 g dlo Ti 3.66 g Cl, 2.77 g Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K 0 Explanation Check All Righes Reserved Terms of Use Pivacy Center Accessib P Type here to search A a Pm Sc T Y P R U DE K A. Alt Ctrl Alt alexa INALED B.arrow_forwardThe equilibrium constant, Kc, for the following reaction is 7.00 × 10-5 at 673 K. NH₂I(s) NH3(g) + HI(g) If an equilibrium mixture of the three compounds in a 5.72 L container at 673 K contains 2.94 mol of NHI(s) and 0.409 mol of NH3, the number of moles of HI present is mol.arrow_forward4arrow_forward
- Sulfur dioxide and oxygen react to form sulfur trioxide, like this: 2 SO₂(g) + O₂(g) → 2 SO3(g) Also, a chemist finds that at a certain temperature the equilibrium mixture of sulfur dioxide, oxygen, and sulfur trioxide has the following composition: compound concentration at equilibrium SO₂ 0.78 M 0₂ SO 3 0.41 M K = [] 0.72 M Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits.arrow_forwardQUESTION#1: TOPIC: Calculating an equilibrium constant from a heterogeneous equilibrium composition Nickel carbonyl decomposes to form nickel and carbon monoxide, like this: NiCO4(g)→Ni(s)+4CO(g) At a certain temperature, a chemist finds that a 2.9 L reaction vessel containing a mixture of nickel carbonyl, nickel, and carbon monoxide at equilibrium has the following composition: compound amount NiCO4 0.792 g Ni 71.9 g CO 1.17 g Calculate the value of the equilibrium constant Kc for this reaction. Round your answer to 2 significant digits. Kc= ? QUESTION#2: TOPIC: Calculating the pH of a strong acid solution A chemist dissolves 185. mg of pure hydroiodic acid in enough water to make up 300. mL of solution. Calculate the pH of the solution. Round your answer to 3 significant decimal places. ?arrow_forwardNickel and carbon monoxide react to form nickel carbonyl, like this: Ni(s)+4 CO(g)→Ni(CO),(9) At a certain temperature, a chemist finds that a 3.4 L reaction vessel containing a mixture of nickel, carbon monoxide, and nickel carbonyl at equilibrium has the following composition: compound amount Ni 88.8 g CO 1.63 g Ni(CO), 0.813 g Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits. K = 0 %3D Check Explanation Privacy Accessibility 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use MacBook Airarrow_forward
- PLEASE HELParrow_forwardIron(III) oxide and hydrogen react to form iron and water, like this: Fe,O3(s)+3 H2(9)–→2 Fe(s)+3 H,O(g) At a certain temperature, a chemist finds that a 4.5 L reaction vessel containing a mixture of iron(III) oxide, hydrogen, iron, and water at equilibrium has the following composition: compound amount Fe,O3 3.54 g H2 3.20 g Fe 4.49 g H,O 1.06 g Calculate the value of the equilibrium constant K_ for this reaction. Round your answer to 2 significant digits.arrow_forwardTitanium(IV) chloride decomposes to form titanium and chlorine, like this: TİCL,(1)→Ti(s)+2 Cl,(9) At a certain temperature, a chemist finds that a 3.4 L reaction vessel containing a mixture of titanium(IV) chloride, titanium, and chlorine at equilibrium has the following composition: compound amount TiCl, 3.86 g Ti 2.68 g Cl2 1.40 g Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K_ = ] x10arrow_forward
- The equilibrium constant for the following reaction is 0.18 at a set temperature. Find the equilibrium concentrations if the initial concentration of PCl3 is 0.225 mol/L and the initial concentration of Cl2 is 0.150 mol/L. PCI3(g)+CI2(g)=PCI5(g)arrow_forwardConsider the following reaction where Kc = 7.00 × 10-5 at 673 K. NH₂I(s) NH3(g) + HI(g) A reaction mixture was found to contain 0.0532 moles of NHI(s), 0.00571 moles of NH3(g), and 0.00837 moles of HI(g), in a 1.00 liter container. Is the reaction at equilibrium? If not, what direction must it run in order to reach equilibrium? The reaction quotient, Qc, equals The reaction must run in the forward direction to reach equilibrium. © must run in the reverse direction to reach equilibrium. O is at equilibrium.arrow_forward#8arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY