
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
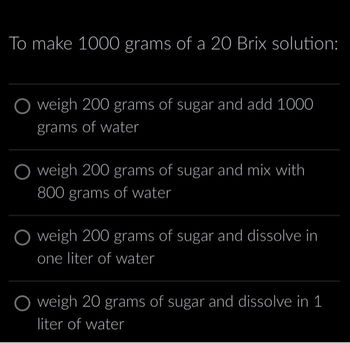
Transcribed Image Text:To make 1000 grams of a 20 Brix solution:
O weigh 200 grams of sugar and add 1000
grams of water
O weigh 200 grams of sugar and mix with
800 grams of water
O weigh 200 grams of sugar and dissolve in
one liter of water
weigh 20 grams of sugar and dissolve in 1
liter of water
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A bottle of champagne is 11%% (vv/vv) alcohol. If there are 500 mLmL of champagne in the bottle, how many milliliters of alcohol are present?arrow_forwardHow many milliliters of a 0.900%0.900% (m/v) normal saline solution can be prepared from 3.003.00 g of sodium chloride, NaClNaCl? Note that mass is not technically the same as weight, but the abbreviation % (w/v) is often used interchangeably with % (m/v).arrow_forwardWhich of the following would not be considered a solution? a) Pepto-bismol O b) Normal Saline IV drip O c) Bronze d) Cоca-cola O e) Airarrow_forward
- How many mililiters of sterile water must be mixed with 70% dextrose to make a total volume of 50 ml of 50% dextrose?arrow_forwardHow many milliliters of a 1:50 (wv) stock solution should be used to make 3 liters of a 1:5000 (w/v) solution?arrow_forwardJamel is to receive Cleocin 275 mg IV for 6 hr. The concentration of the solution is 5.0 mg/mL. How many milliliters of solution will you need to deliver?arrow_forward
- What volume (mL) of a 15% (m/v) NaOH solution contains 120 g NaOH? 120 mL 13 mL 8.0 x 10² mL 0.13 mL 18 mLarrow_forwardHow many milliliters of a 0.900% (m/v) normal saline solution can be prepared from 3.50 g of sodium chloride, NaCl? Note that mass is not technically the same as weight, but the abbreviation % (w/v) is often used interchangeably with % (m/v). volume: mLarrow_forwardWhat is the volume in milliliters of a 85% (v/v) rubbing alcohol solution that contains 15 mL of alcohol? 7.0 x 103 mL O 5.7 x 102 mL O 1.8 x 101 mL O 5.7 x 102 mL Next hparrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY