
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
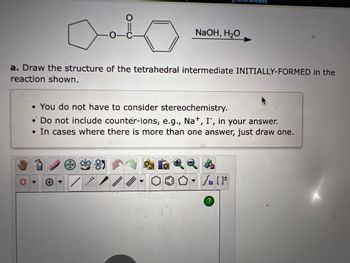
Transcribed Image Text:0
a. Draw the structure of the tetrahedral intermediate INITIALLY-FORMED in the
reaction shown.
-o-c-
*
• You do not have to consider stereochemistry.
• Do not include counter-ions, e.g., Na+, I, in your answer.
• In cases where there is more than one answer, just draw one.
●
NaOH, H₂O
9-85
11***
√ [F
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 17. a. Label the reactive features, highlight the most reactive one, then highlight what it needs. Also, state if the reaction will start to create a carbocation, carbon radical, or carbanion, or will cause loss of aromatic character. If a carbocation, carbon radical, or carbanion starts to develop, label where that will occur. -CH3 with H,SO, b. Use mechanism arrows to illustrate the reaction that occurs. c. If applicable, use stabilization resources to deal with the carbocation, carbon radical, or carbanion that starts to develop during the reaction, and draw the structure of any resonance-stabilized intermediate. d. Continue labeling and diagramming the reaction until you find the major stable product(s). e. Finally, state the stereochemistry of the major product(s) and use either Fisher projection or perspective formula representations to illustrate that stereochemistry.arrow_forwardFill in the missing product, or reactant in the reactions below. If no reaction will happen, write “NOREACTION” in the product box. If multiple products can form, show the MAJOR product, taking care toillustrate stereochemistry as appropriatearrow_forwardFor the dehydrohalogenation (E2) reaction shown, draw the major organic product, including stereochemistry. (CH,),COK y (CH,),COH Incorrectarrow_forward
- 3) Draw the MAJOR organic products for the following reactions. Include stereochemistry and diastereoisomers if they result. If there are enantiomers do not worry about drawing other enantiomer, just write plus enantiomer. If a reaction does not occur simply write no reaction (8 pts). a. Br₂, H₂O b. C. d. 1. 03 2. DMS KMnO4, NaOH mCPBAarrow_forwardFill in the information based on the type of reaction mentioned in each column. A. Draw the line angle diagram of a substrate that will undergo SN1, SN2, E1, E2 B. Draw the line angle diagram of a nucleophile that can be used for SN1, SN2, E1, and E2 C. Draw the line angle diagram of a solvent that can be used with SN1, SN2, E1, and E2 D. Write down if the stereochemistry of the product will be inversion, retention, or a 50:50 ratio of inversion and retentionarrow_forwardDraw the product(s) of the following reactions. CH3CH₂CH₂-CEC-CH₂ (+) Consider E/Z stereochemistry of alkenes. • Separate multiple products using the + sign from the drop-down menu. • If no reaction occurs, draw the organic starting material. still Na/NH3(1) t-BuOH ChemDoodleⓇ Ⓒ ار ۵۰ ]arrow_forward
- B 10. For the cycloaddition reaction shown below a) Give the curved arrow-pushing and draw the allowed product for the reaction, be sure to include stereochemistry and indicate any racemic mixtures A heat b) on TOP of reactant A draw a pictorial representation of the HOMO (draw it on the structure like the one shown below rather than the one in the equation so that the curved arrow-pushing isn't obscured) B c) on TOP of reactant B draw a pictorial representation of the LUMO (draw it on the structure below rather than the one in the equation so that the curved arrow-pushing isn't obscured) d) Is the allowed reaction suprafacial or antarafacial for reactant A ? e) Is the allowed reaction suprafacial or antarafacial for reactant B? 5arrow_forwardFor the dehydration shown, use curved arrows to show the formation of the carbocation intermediate in the presence of sulfuric acid H, SO,, then draw the structures of the minor and major products of the elimination. 4 H2SO4 Major and Minor products HO: The H, SO, is abbreviated as H* in the drawing module. Do not delete any pre-drawn bonds, charges, or lone pairs. If you 4 accidentally make a mistake, remove the last change by using the undo button on the lower left or revert the drawing palette to the original state by selecting the More menu, then select Reset Drawing. Step 1: Use curved arrows to complete the Step 2: Use a curved arrow to show the protonation mechanism of the alcohol. formation of the carbocation intermediate for the elimination. Select Draw Rings More Erase Select Draw Rings More Erase C H +H, SO, : OH он,arrow_forwardA. Predict the major product of the reaction. Clearly indicate stereochemistry, if necessary. 1. NaNH2 (excess) NH3/THF Br Br i-Pr 2. H3O* B. Draw a detailed arrow-pushing mechanism for the transformation, accounting for stereochemistry, if necessary.arrow_forward
- Draw a structural formula for the major organic product of the reaction shown ل ساز اسامة CH3 8 . Consider E/Z stereochemistry of alkenes. • Do not show stereochemistry in other cases. • You do not have to explicitly draw H atoms. • Do not include organocopper or inorganic ion by-products in your answer. II... 2 Y CuLi {n [F ChemDoodle Previous Next Save and Exitarrow_forwardDraw the major organic product(s) of the following reaction. 0 . You do not have to consider stereochemistry. • If no reaction occurs, draw the organic starting material. II... + • When SN1 & E1 pathways compete, show both the substitution and the elimination products. Separate multiple products using the + sign from the drop-down menu. . Do not include counter-ions, e.g., Na*, I, in your answer. /// ? H₂O ChemDoodlearrow_forward1. Pick One: Draw one of the two mechanisms shown below. You may draw both for practice. Give the complete, detailed mechanism for the following reaction. Be sure to include an explanation of the region chemistry and stereochemistry. What type of reaction is this? What is the stereochemistry of the product? CH H. H,0 CIarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY