
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
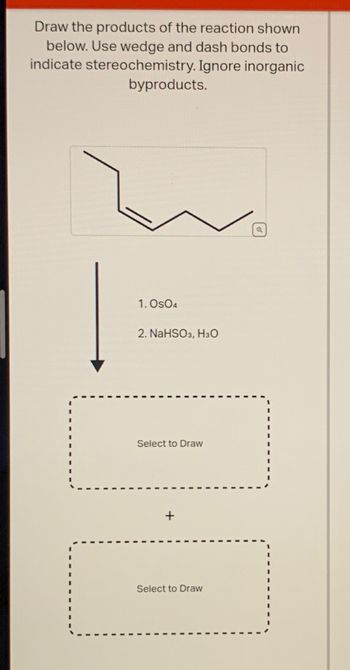
Transcribed Image Text:Draw the products of the reaction shown
below. Use wedge and dash bonds to
indicate stereochemistry. Ignore inorganic
byproducts.
1. Os04
2. NaHSO3, H3O
Select to Draw
Select to Draw
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- tu Please don't provide handwritten solutionarrow_forwardDraw the full electron pushing mechanism for the reaction depicted including ALL intermediates (with lone pairs and formal charges). Clearly label the electrophile and nucleophile in each step where appropriate.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts and the alkoxide side product. N. H 1. excess LIAIH4 2. H2O Type here to searcharrow_forward
- Draw the least stable resonance form for the intermediate in the following electrophilic substitution reaction. CF3 CF3 Br2/FeBr3 Br . You do not have to consider stereochemistry. • Include all valence lone pairs in your answer. • In cases where there is more than one answer, just draw one. n ?arrow_forwardDraw the major product(s) of the following reaction.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. 1. CH3MgBr (excess) 2. H₂O Drawing CI >arrow_forward
- Draw all intermediates and show all electron - pushing OH HBr A. - Br B. 오이 CH3NH2 C. H₂O® E. NaOEt HOEt S D. OHarrow_forwardDraw the strcutres of the missing reactants, intermediates, or products in the following mechanisms. Include all lone pairs.arrow_forwardDraw the alkene that would react with the reagent given to account for the product formed. ? + + H₂O **** H₂S04 • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. CH3 CH3 CHCCH3 | | OH CH3 +1arrow_forward
- Helparrow_forward027 In Part 1 add the curved arrows to the nucleophilic acyl substitution reaction mechanism. In Part 2, answer the multiple-choice question about the reaction in Part 1. Part 1 4 See Periodic Table O See Hint :0: :Br: Add the missing curved arrow notation. S CI Br Part 2 Which statement is most correct regarding the equilibrium for the above reaction? Choose one: O The equilibrium favors the right (i.e., Kea > 1) because acetate is a better leaving group than bromide. O The equilibrium favors the right (i.e., Kea > 1) because bromide is a better leaving group than acetate. O The equilibrium favors the left (i.e., Keq« 1) because acetate is a better leaving group than bromide. O The equilibrium favors the left (i.e., Keg 1) because bromide is a better leaving group than acetate. +arrow_forwardquestion 5 helparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY